N |
您所在的位置:网站首页 › 脱boc生成什么 › N |
N
|
【C. Jaume; G. G.Santiago et al., Tetrahedron: Asymmetry,2000, 11(22),4549-4458】
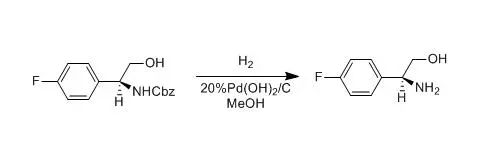
A solution of N-Cbz arylglycinol (17) (1.02 mmol) in MeOH (10 mL) wasstirred for 15 min in the presence of an excess of Pd(OH)2/C under a dihydrogenatmosphere. The solution was then filtered on a Celite pad and the solvent removedin vaccuo. Purification of the crude afforded the desired free 2-arylglycinols(S)-21 in 87% yield, white solid;[a]D20=+47.0 (c=0.78,CHCl3); mp 94-96°C(AcOEt)。 【B.Pierfrancesco; C. silvia et al., Tetrahedron,1999, 55(10),3025】
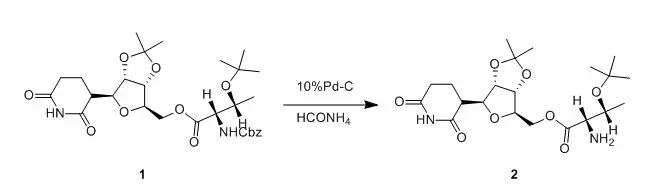
576.6mg of compound 1 (1 mmol) was dissolved in 20 ml of methanol. Then 150 mg ofammonium formate (3 mmol) and 75 mg of 10% Pd-C was added and the reactionmixture was stirred at room temperature 10 min and then heated to reflux for 45min. The mixture was filtered through celite and the filtrate was evaporate todryness to give 430 mg of compound 2 (98%). This compound was used without further purification in the subsequentstep. 【Alargov, D. K; Naydenova, Z; Monatsh. Chem., 1997, 128(6-7),725-732】
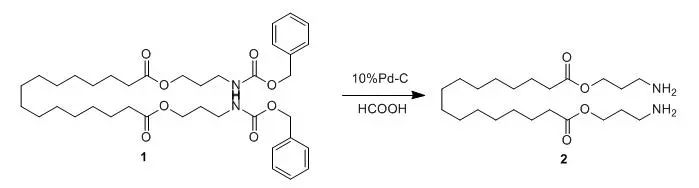
Compound 1(0.6 g, 0.8 mmol) was dissolved in 1:1 formicacid/methanol (60 mL) and added to a round-bottom flask (100 mL) containing 1equiv of palladium catalyst (10% Pd/C, 1.0 g, 0.9 mmol). The mixture was continuously stirred under refluxtemperature for 24 h. The catalyst was removed by filtration and washed with anadditional 10 mL of methanol. The combined solvents were removed by evaporationunder reduced pressure to give Compound 2(0.34 g, 81%, a white solid, mp 96-98 °C). This compound was used withoutfurther purification in the subsequent step. 【Fyles, T. M.; Zeng, B.; J. Org. Chem., 1998, 63(23), 8337-8345】
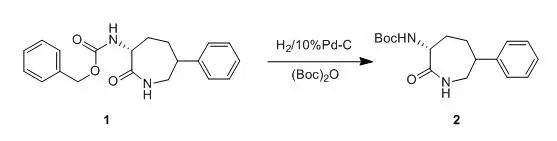
10%Pd-C wasaddede to a solution of compound 1 (596 mg , 1.77 mmol) and (Boc)2O(773 mg, 3.54 mmol) in etnyl acetate (30 ml). The reation vessel was evacuatedand back-filled with nitrogen (three times), then back-filled with hydrogen (1atm). After 2 h, the mixture was filtered and concentrated. Purification bysilica gel chromatography (30% ethyl acetate/ hexanes - 50% ethyl acetate/hexanes) gave compound 2 (289 mg, 54%). 【WO2004092166】
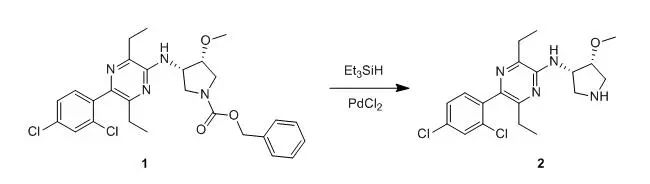
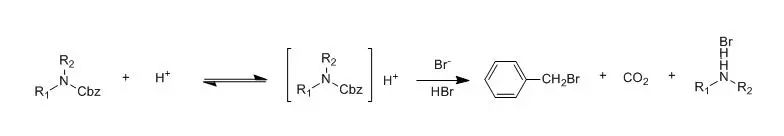
To a solution ocompound 1(900 mg) in methylene chloride (16.5 ml) was addede PdCl2 (30mg) and triethylamine (0.229 ml). Triethyl silane was added (2 x 0.395 ml) over2 h. The reaction mixture stirred 1 h and 2 ml of trifluoroacetic acid wasadded. After 30 min the reaction was basified with 2 N NaOH, extracted with methylene chloride, dried over MgSO4,filtered and concentrated. Chromatography was run on a biotage 40S column with3-5% MeOH/CH2Cl2 with 0.5% NH4OH to provide compound 2as a oil (501mg, 74%). 【US20030144297】 酸解脱除 氨基甲酸苄酯在强酸性条件下容易去保护。HBr/HOAc 是酸解脱除苄氧羰基的最常用的试剂。脱除反应主要按下式进行。反应需要消耗2分子的HBr,Cbz的脱除速度随HBr浓度的增大而增大,因此实际上都是采用高浓度的过量HBr/HOAc溶液(1.2M-3.3M)以保证反应的完全。
含有丝氨酸和苏氨酸的肽或其它含羟基的氨基衍生物用HBr/HOAc脱除Cbz时会发生羟基的O-乙酰化反应。虽然O-乙酰基能用碱皂化或氨解脱去,但为了避免这个副反应,可以改用HBr/二氧六环或HBr/三氟乙酸来代替HBr/HOAc。由于HBr在三氟乙酸中的溶解度较小,因此不能预先制成HBr/三氟乙酸溶液,而只能将保护的肽或氨基衍生物溶于无水三氟乙酸中,先于0℃下通入干燥的HBr,待Cbz大部分脱除后,再室温通短时间以求完全脱除变化基。Cbz被HBr分解产生的溴化苄能同肽中的某种氨基酸反应,也是需要加以注意的。如,甲硫氨酸的硫原子能同溴化苄反应生成S-苄基甲硫氨酸,防止的办法是加入硫醚(CH3SC2H5)为捕捉剂。色氨酸被HBr/HOAc分解产生有色物质,防止的办法是加入亚磷酸二乙酯。硝基精氨酸会发生硝基的部分脱落,改用液体HBr于-67℃处理可以避免。 用液体HF在0℃处理10-30分钟即可将Cbz完全脱去。FSO3H、CH3SO3H、CF3SO3H和C6H5SCH3-TFA也是较好的试剂。Me3SiI在氯仿、乙腈中能于几分钟内选择性脱去Cbz和Boc保护基。对于BBr3/CH2Cl2而言,较大分子的肽的Cbz衍生物可在TFA中去除,因为肽在酸中的溶解度比在CH2Cl2中大。从肽中脱去Cbz,可在TFA中添加0.5 M4-(甲硫基)苯酚或使用HF/Me2S/对甲苯酚(25:65:10,v/v)来抑制Bn+对芳香氨基酸的加成。 此外,已经报道过的还有以下的一些不常用的方法。如HCl/CHCl3、HCl/HOAc、HBr/SO2、液体HBr、TosOH、HI/HOAc、碘化磷、Et3SiH、沸腾的TFA、8MHCl的乙醇液或6 MHCl回流1小时或浓盐酸于25-75℃加热处理1-1.5小时等。
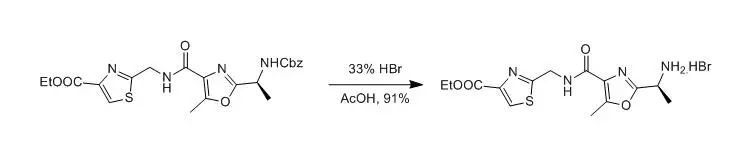
A solution of the amine Cbz compund (208 mg, 0.44mmol) in 33 % hydrobromic acid in acetic acid (1 mL) and glacial acetic acid(0.6 mL) was stirred at rt for 3 h under an atmosphere of nitrogen. Thevolatiles were removed in vacuo to leave the free amine hydrobromide (168mg, 91 %) as a brown, highly hygroscopic powder; [α]D=-18.0° (c = 0.4, EtOH) 【B.Anna; P. Gerald., Heterocycles, 2002, 58, 521】
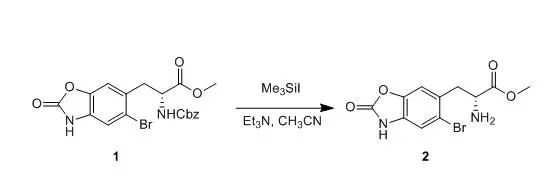
Me3SiI (0.73 ml,0.73 mmol) was added to a soluton of compound 1(146 mg, 0.33 mmol) inacetonitrile (10 ml) at room temperature, and the resulting mixture was stirredat room temperature for 2 h. Et3N (0.12 ml) was added and themixture was stirred at room temperature for 15 min. The solvents were removedin vacuo, and the residue was extracted with ethyl acetate. The combinedorganics were washed with sodium bicarbonate and brine, dried over sodiumsulfate and filtered. Solvents were removed and the residue was used directlyin the next step. 【US20040204397】 本文非原创内容,版权归原作者所有,如涉及版权问题,请联系本号删除。返回搜狐,查看更多 |
【本文地址】
今日新闻 |
推荐新闻 |