叔丁氧羰基(Boc)保护胺基 |
您所在的位置:网站首页 › 脱boc反应条件 › 叔丁氧羰基(Boc)保护胺基 |
叔丁氧羰基(Boc)保护胺基
|
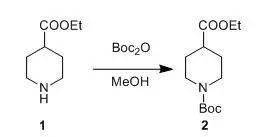
二、 Boc酸酐在甲醇中与胺直接反应
Boc2O (262 g, 1.2 mol) in MeOH (250 ml) was added toa soluton of compound 1 (157.2 g,1.0 mol) in MeOH (350 ml) at 10°C,and the resulting mixture was stirred at room temperature for 2 h. N1,N1-dimethylethane-1,2-diamine (26 g, 0.3 mol) was added and the mixture was stirredat room temperature for 15 min. The solvent was removed in vacuo, and theresidue was dissolved with ethyl acetate (750 ml). The combined organics werewashed with 1 N HCl (2 x 250 ml) and brine (2 x 250 ml), dried over sodiumsulfate and filtered. The solvent was removed to give compound 2 (250 g, 96%), which was used directly in thenext step. 三、芳胺的单Boc保护示例 3-Aminopyridine-2-carboxylic acid(5.02 g, 36 mmol) was suspendedin 60 mL of dry DMF, and Et3N (15.2 mL, 108 mmol) was added dropwise at roomtemperature. To the resulting brown solution was added Boc2O (11.80 g, 54 mmol). After being stirred for 10min, the mixture was heated at 40-50 °Covernight. The reaction mixture was poured into water and was then extractedwith EtOAc (2 X 50 mL). The aqueous phase was acidified to pH 4-5 with 2 M aqueous HCl and then extracted with CH2Cl2(3 X 50 mL). The combined organic phases were thenprocessed in the usual way and chromatographed (13:1 CHCl3/MeOH) to yieldthe desired product(4.2 g,49%). 四、 芳胺的双Boc保护示例
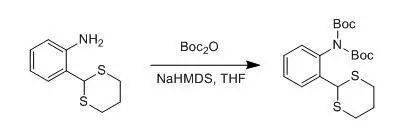
Macleod, Calim;Mckieman, Gordon J et al., J. Org. Chem., 2003, 68(2) ,387-401 A solution of NaHMDS (22.0 mL, 22.0 mmol, 1 M in THF) was added to a solution of the amine (2.11 g, 10.0 mmol) and (Boc) 2O (5.46 g, 25.0 mmol) in THF (50 mL) at 0°C under nitrogen. The reaction was allowedto warm to rt and stirred for 16 h. After this time, the reaction was poured intowater, extracted into CH2Cl2(2 X 25 mL),washed with water (2 X 25 mL), dried over Na2SO4, and concentrated to yield a white-yellowsolid. Recrystalization from petroleum ether (40-60 °C) gave the imide as needles (3.21 g, 7.80 mmol, 78%). R f (hexane/ CH2Cl21:9, SiO2): 0.10. Mp:106-109 °C. 五、 酰胺的Boc保护示例
Lars G. J. Hammarström, Yanwen Fuet al., Org. Syn.,81 , 213 A 2000-mL, three-necked, round-bottomed flask equippedwith an argon inlet adapter, glass stopper, and an overheadmechanical stirrer is charged with a suspension of the hydantoin 1(26.0 g,154 mmol) in 1000 mL of 1,2-dimethoxyethane. Triethylamine (15.7 g, 154mmol) is added in one portion, and the resulting white suspension isstirred for 30 min. Di-tert-butyl dicarbonate (168.0 g, 770mmol) is then added by pipette, followed by4-dimethylaminopyridine (DMAP)(0.2 g, 1.5 mmol). Six additional 0.2 g-portionsof DMAP are added at 12 hr intervals during the course of the reaction.The reaction mixture is stirred vigorously for a total of 72 hr, and the resultinglight yellow solid is then collected in a Büchner funnelusing suction filtration. The filtrate is concentrated to a volume of 60 mL byrotary evaporation, and the resulting solution is cooled to 15°C. The precipitate which appears iscollected using suction filtration, added to the first crop, and the combinedsolids are dissolved in 500 mL of chloroform. This solution is washed with three 200-mL portions of 1.0N HCl, and the combinedaqueous phases are extracted with 100 mL of chloroform. The combined organic layers are washed with 100 mL of saturated aq NaHCO 3solutionand 100 mL of brine,dried over anhydrous MgSO4,filtered, and concentrated by rotary evaporation. The resulting solid is driedat room temperature at 0.01 mmfor 24 hr. The resulting finely ground light yellow solid is suspended in 400 mL of diethyl ether in a 1000-mL, round-bottomed flask equipped with a magnetic stirbar, stirred for 2 hr, and filtered on aBüchner funnel washing with four 50-mL portions of diethyl ether. The product is dried under vacuum (85°C; 0.5 mm) for 24 hr to give 60.0–65.3 g (83-90%)of 2as a ivory-colored solid. 六、吲哚Boc保护示例
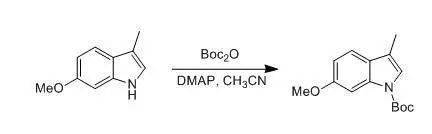
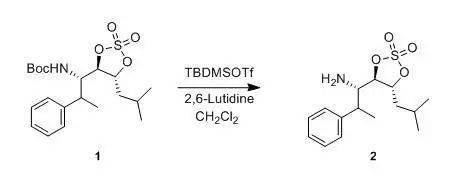
G. Tong; P. Ruiyan et al., J. Org.Chem., 1997, 26, 9298 To a solution of6-methoxy-3-methylindole (5.0 g,31 mmol) in distilled acetonitrile (150 mL) were added di- tert butyl dicarbonate(7.44 g, 34.1 mmol) andDMAP (0.195 g, 1.6 mmol).The reaction mixture was stirred at rt for 12 h. The solvent was removed underreduced pressure. The residue was dissolved in CH 2Cl2(100 mL) and washed with an aqueous solution of 1 N HCl (2 x 50 mL). Theaqueous layer was extracted with CH2Cl2(3 x 30 mL). The combined organic ayers were dried (K2CO3). Afterremoval of solvent under reduced pressure, the residue was solidified to affordthe product (8.12 g, 99%) as a yellow solid: mp 45-46 °C. Boc去保护 Boc比Cbz对酸敏感,酸解产物为异丁烯和CO 2 (见下式)。在液相肽的合成中,Boc的脱除一般可用TFA或50%TFA(TFA:CH 2 Cl 2 = 1:1,v/v )。而在固相肽合成中,由于TFA会带来一些副反应(如在得到的胺上上一个三氟乙酰基等),因此多采用1-2MHCl/有机溶剂。一般而言用HCl/二氧六环,比较多见。 用甲醇作溶剂,HCl/EtOAc的组合使TBDMS和TBDPS酯[ 1] 以及叔丁酯和非酚类酯在Boc脱除时不被断裂,而S-Boc除外[ 2] 。但当同时脱除分子中Boc和叔丁酯, 或分子中有游离羧酸基,千万记住不能用HCl/MeOH,其可将羧酸变为甲酯。同时AcCl/MeOH,则是一个在甲醇中产生无水HCl的便利方法。这些条件也可用来从羧酸制备酯以及形成胺的盐酸盐[ 3]。 在中性的无水条件下Me 3 SiI 在CHCl 3 或CH 3 CN 中除了能脱除Boc外,也能断裂氨基甲酸酯、酯、醚和缩酮。通过控制条件可以得到一定的选择性[ 4] 。 当分子中存在一些官能团其可与副产物叔丁基碳正离子在酸性下反应时,需要添加硫酚(如苯硫酚)来清除叔丁基碳正离子,如此举可防止蛋氨酸和色氨酸的脱Boc时的烷基化[ 5] 。也可使用其它的清除剂,如苯甲醚、苯硫基甲醚、甲苯硫酚、甲苯酚及二甲硫醚[ 6] 。在Boc脱去过程中TBDPS [ 7]和TBDMS [ 8]基对CF 3 COOH 是稳定的(在TBS存在,用相对稀一些的10-20%TFA)。伯胺衍生物存在下,ZnBr 2 /CH 2 Cl 2 可以选择性的脱除仲胺上的Boc [ 9] 1. F. Cavelier, C.Enjabal., Tetrahedron Lett., 1996, 37 , 5131 2. F. S. Gibson,S. C. Bergmeier, H. Rapoport., J. Org. Chem.,1994, 59, 3216 3. A.Nudelman, Y.Bechor et al., Synth. Commun., 1998, 28 , 471 4. R. S. Lott, V.S. Chauhan et al., J. Chem. Soc. Chem. Commun., 1979, 495; G. A.Olah, S. C. Narang., Tetrahedron., 1982, 38 , 2225 5. R. A. T. M. vanBenthem, H. Hiemstra et al., J. Org. Chem.,1992, 57, 6083 6. M. Bodanszky,A. Bodanszky., Int. J. Pept. Protein Res. , 1984, 23 , 565;Y. Masui, N. Chino et al., Bull. Chem. Soc. Jpn., 1980, 53 ,464 7. P. A. Jacobi,S. Murphree et al., J. Org. Chem.,1996, 61, 2413 8. J. Deng, Y.Hamada et al., J. Am. Chem. Soc., 1995, 117 , 7824 9. S. C. Nigam, A. Mann et al., Synth. Commun., 1989, 19 , 3139 反应实例 一、TMSOTf中性条件下脱Boc示例 To a solution containing 2 (1.0 g, 3.9 mmol) in 30 mL of dry CH2Cl2was slowly added TBDMSOTf (0.9 mL, 4.1 mmol). After stirring the reactionmixture for 6 h, the solvent was evaporated, and the crude product (0.8 g, 75%) was obtaineded, which was used directly inthe next step. 二、 TMSOTf-2,6-lutidine中性条件下脱Boc示例
To a stirring solution of compound 1 (800 mg, 2.0 mmol) and2,6-lutidine (0.4463 ml, 4.0 mmol) in CH 2Cl2(6 mL) was added tert -butyldimethylsilyl triflate (0.690 ml, 3.0mmol) dropwise over 5 min. After 20 min, saturated NH 4Cl (10 mL) wasadded. The mixture was stirred and separated, and the aqueous layer wasextracted with Et2O (3 x 15 mL). The combined organic layers were washedwith water (2 x 10 mL) and saturated NaCl (10 mL), dried (MgSO4), andconcentrated to give the crude silyl carbamate, which was dissolved inTHF (10 mL) and cooled to 0°C.A 1.0 M solution of TBAF in THF(2 mL, 2 mmol) was added over 5 min, and then the solution was stirred at 0°C for 1 h. The solution was concentratedand chromatographed (95:5 CH 2Cl2-methanol) through a small plug of silica to give compound 2(882 mg, 75%) as a clear oil. 三、TFA脱Boc示例 M. Alberto; A. Eduardo et al., J. Org. Chem., 2004, 21, 7004 To a solution ofthe β-aminoester (0.2 mmol) in CH 2 Cl 2 (3 mL), cooled to 0°C was added TFA (1mL). After the consumption of the starting material (45 min, monitored by TLC), the mixture was evaporated and thensaturated aqueous NaHCO 3 was added. The aqueous layer was extractedtwice with CH 2 Cl 2 (15 mL), and the organic layer washedwith brine and dried over anhydrous Na 2 SO 4 . The solventwas removered under vacuum, to afford the amine, which were employed without further purification to prepare the Mosher’s diastereoisomeric amides. 四、HCl-THF脱Boc示例 J.Wehbe et al., Tetrahedron: Asymmetry,2004, 15 , 851 Tothe Boc protected amine (0.06 g,0.17 mmol) dissolved in THF (1mL) was added 2M HCl (1mL, 2 mmol) and the mixture stirred 2 h atroom temperature. After evaporation of the solvent, the product was extractedinto EtOAc (3.5mL).The organic layer was dried and evaporated under vacuum to afford 17b in 95% yield as a whitesolid. 五、叔丁酯存在下的脱Boc示例1
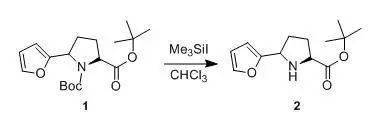
1.77 ml of Me 3 SiIare added dropwise at room temperature in the vicinity of 25 °Cto a soution of 3.8 gof compound 1in50 ml of CHCl 3 . Stirring is contiuned for 30 min, then 20 ml f waterare addede. The aqueous phase is separated, then extracted with CHCl 3 (2x 20 ml). The organic phases are combined, washed successively with a saturatedaqueous Na 2 CO 3 (30 ml) and water(2 x 30 ml), then driedover MgSO 4 and concentrated to dryness under reduced pressure at 40°C. The mixture of the two diastereoisomersobtained is separated by chromatography on silica (eluent: ethylacetate/cyclohexane = 1/4). The fractions containing the expected product arecombined and concentrated to dryness under reduced pressure at 40°C to give compound 2(0.5 g), as a yellow-orange oil, used as it isin subsequent syntheses. 六、叔丁酯存在下的脱Boc示例2
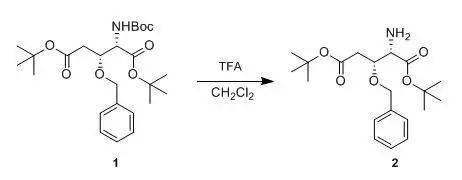
To a solution of compound 1(149 mg, 0.33 mmol) in CH 2 Cl 2 (2 ml), TFA (1 ml) as addedat 0 °Cand the mixture was stirred for 1 h at 0°C. Saturated aqueous Na 2 CO 3 was added and the mixture was etracted with CHCl 3 . The etract waspurified by silica gel column chromatography to obtained compound 2(92mg, 79%). 返回搜狐,查看更多 |
【本文地址】
今日新闻 |
推荐新闻 |