N |
您所在的位置:网站首页 › 脱boc的方法后处理 › N |
N
|
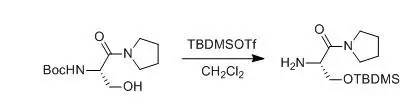
5.R. A. T. M. vanBenthem, H. Hiemstra et al., J. Org. Chem.,1992, 57,6083 6.M. Bodanszky,A. Bodanszky., Int. J. Pept. Protein Res., 1984, 23, 565;Y. Masui, N. Chino et al., Bull. Chem. Soc. Jpn.,1980, 53,464 7.P. A. Jacobi,S. Murphree et al., J. Org. Chem.,1996, 61,2413 8.J. Deng, Y.Hamada et al., J. Am. Chem. Soc.,1995, 117, 7824 9.S. C. Nigam, A. Mann et al., Synth. Commun.,1989, 19, 3139 反应实例 一、TMSOTf中性条件下脱Boc示例
Gilbertson, Scott R; Chang, Cheng-Wei et al., J.Org. Chem.,1998, 63(23), 8424-8431 To a solution containing 2 (1.0 g, 3.9 mmol) in 30 mL of dry CH2Cl2was slowly added TBDMSOTf (0.9 mL, 4.1 mmol). After stirring the reactionmixture for 6 h, the solvent was evaporated, and the crude product (0.8 g, 75%) was obtaineded, which was used directly inthe next step. 二、 TMSOTf-2,6-lutidine中性条件下脱Boc示例
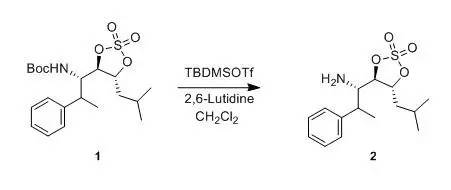
Kemp, Scott J; Bao, Jiaming et al J. Org.Chem.,1996, 61(20), 7162-7167 To a stirring solution of compound1 (800 mg, 2.0 mmol) and2,6-lutidine (0.4463 ml, 4.0 mmol) in CH2Cl2(6 mL) was added tert-butyldimethylsilyl triflate (0.690 ml, 3.0mmol) dropwise over 5 min. After 20 min, saturated NH4Cl (10 mL) wasadded. The mixture was stirred and separated, and the aqueous layer wasextracted with Et2O (3 x 15 mL). The combined organic layers were washedwith water (2 x 10 mL) and saturated NaCl (10 mL), dried (MgSO4), andconcentrated to give the crude silyl carbamate, whichwas dissolved inTHF (10 mL) and cooled to 0°C.A 1.0 M solution of TBAF in THF(2 mL, 2 mmol) was added over 5 min, and then the solution was stirred at 0°C for 1 h. The solution was concentratedand chromatographed (95:5 CH2Cl2-methanol) through a small plug of silica to give compound2(882 mg, 75%) as a clear oil. 三、TFA脱Boc示例
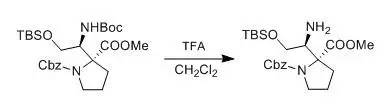
M. Alberto; A. Eduardo et al., J. Org. Chem.,2004, 21,7004 To a solution ofthe β-aminoester (0.2 mmol) in CH2Cl2 (3 mL), cooled to 0°C was added TFA (1mL). After the consumption of the starting material (45 min, monitored by TLC), the mixture was evaporated and thensaturated aqueous NaHCO3 was added. The aqueous layer was extractedtwice with CH2Cl2 (15 mL), and the organic layer washedwith brine and dried over anhydrous Na2SO4. The solventwas removered under vacuum, to afford the amine, which were employed without further purification to prepare the Mosher’s diastereoisomeric amides. 四、HCl-THF脱Boc示例
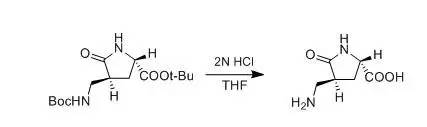
J.Wehbe et al., Tetrahedron: Asymmetry,2004, 15, 851 Tothe Boc protected amine (0.06 g,0.17 mmol) dissolved in THF (1mL) was added 2M HCl (1mL, 2 mmol) and the mixture stirred 2 h atroom temperature. After evaporation of the solvent, the product was extractedinto EtOAc (3.5mL).The organic layer was dried and evaporated under vacuum to afford 17b in 95% yield as a whitesolid. 五、叔丁酯存在下的脱Boc示例1
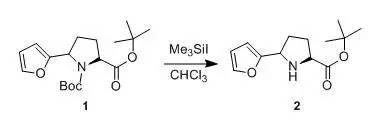
US5610144 1.77 ml of Me3SiIare added dropwise at room temperature in the vicinity of 25°Cto a soution of 3.8 gof compound 1in50 ml of CHCl3. Stirring is contiuned for 30 min, then 20 ml f waterare addede. The aqueous phase is separated, then extracted with CHCl3(2x 20 ml). The organic phases are combined, washed successively with a saturatedaqueous Na2CO3(30 ml) and water(2 x 30 ml), then driedover MgSO4 and concentrated to dryness under reduced pressure at 40°C. The mixture of the two diastereoisomersobtained is separated by chromatography on silica (eluent: ethylacetate/cyclohexane = 1/4). The fractions containing the expected product arecombined and concentrated to dryness under reduced pressure at 40°C to give compound 2(0.5 g), as a yellow-orange oil, used as it isin subsequent syntheses. 六、叔丁酯存在下的脱Boc示例2
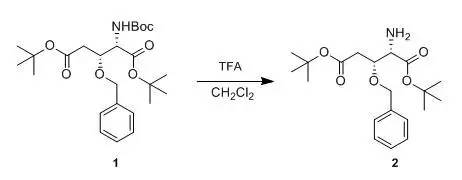
WO20040106286 To a solution of compound 1(149 mg, 0.33 mmol) in CH2Cl2 (2 ml), TFA (1 ml) as addedat 0°Cand the mixture was stirred for 1 h at 0°C. Saturated aqueous Na2CO3was added and the mixture was etracted with CHCl3. The etract waspurified by silica gel column chromatography to obtained compound 2(92mg, 79%). 本文非原创内容,版权归原作者所有。返回搜狐,查看更多 |
【本文地址】