羟醛缩合反应(Aldol condensation) |
您所在的位置:网站首页 › cannizzaro反应与羟醛缩合反应在醛的结构上有何不同 › 羟醛缩合反应(Aldol condensation) |
羟醛缩合反应(Aldol condensation)
|
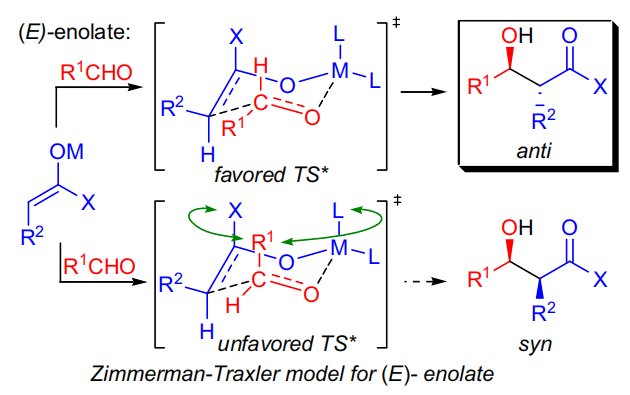
一般而言,使用M-O键强的金属(硬酸,络合能较大的金属)六元环过渡态的环足够稳固,立体选择性会提高。 加入HMPA等与锂等金属配位能力较强的配位性溶剂、可使金属烯醇盐得到极化,提高反应性能。另一方面,因它不可能采取六元环过渡态,会使选择性反转,就变得依赖于底物。 反应实例
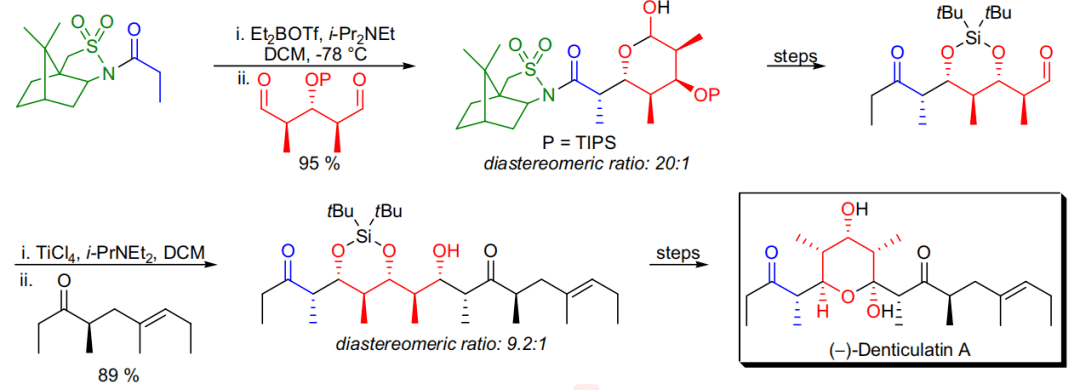
【 Org. Lett. 2003, 5, 733-736】
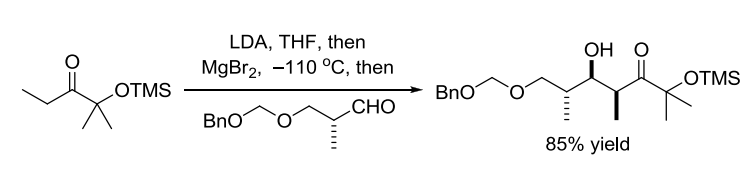
【 Tetrahedron Lett. 1980, 21, 1031-1034】
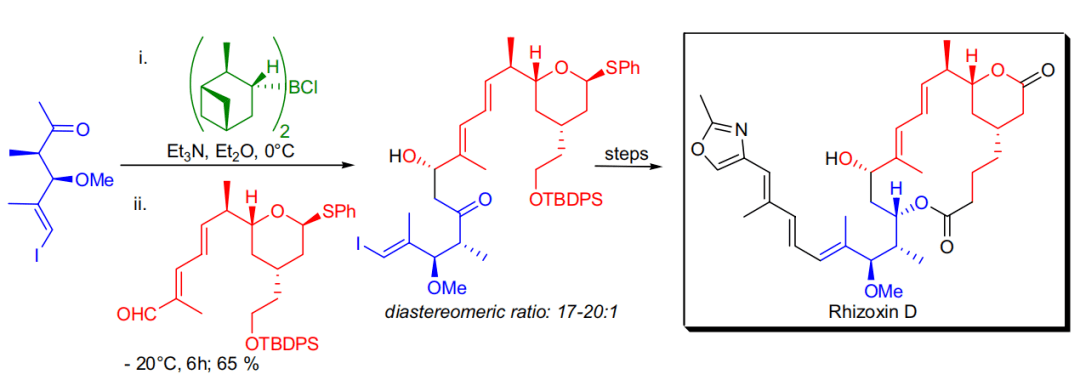
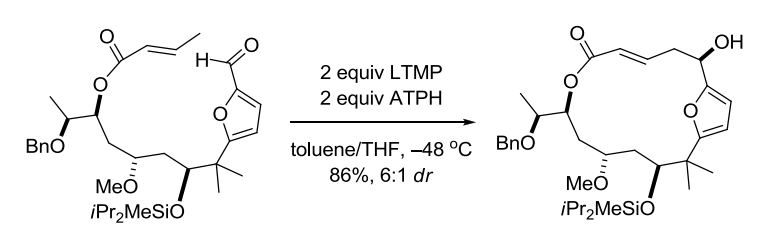
【 Angew. Chem. Int. Ed. 1997, 36, 166-168】
【 Eur. J. Org. Chem. 2008, 1759-1766】
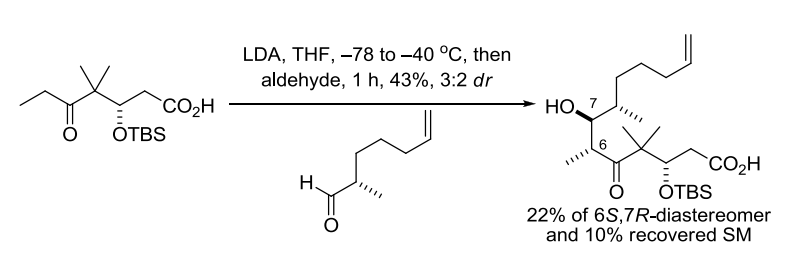
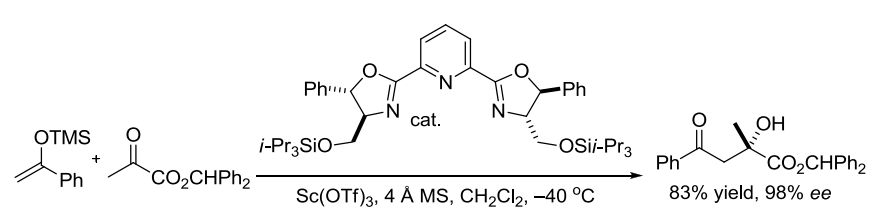
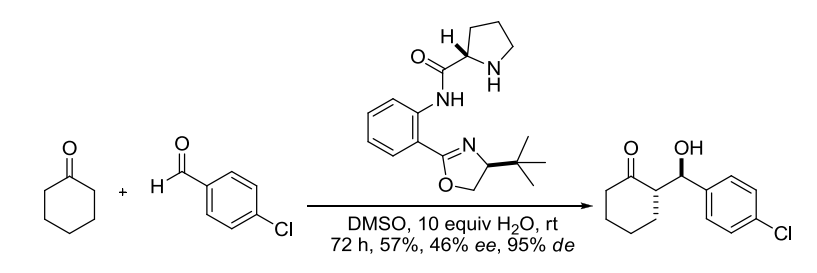
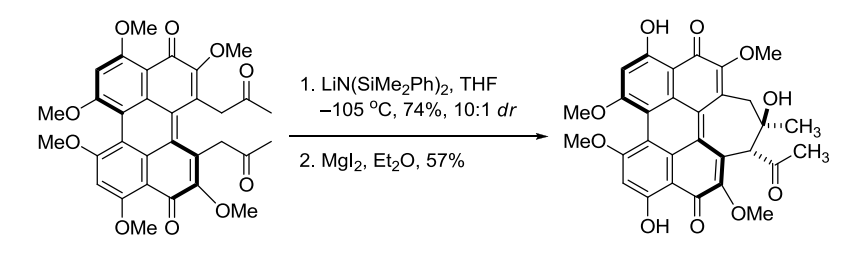
【 Org. Lett. 2012, 14, 178-181】 相关文献 1. Wurtz, C. A. Bull. Soc. Chim. Fr. 1872, 17, 436-442. Charles Adolphe Wurtz(1817-1884) was born in Strasbourg, France. After his doctoral training, he spent a year under Liebig in 1843. In 1874, Wurtz became the Chair of Organic Chemistry at the Sorbonne, where he educated many illustrous chemists such as Crafts, Fittig, Friedel, and van’t Hoff. The Wurtz reaction, where two alkyl halidesare treated with sodium to form a new carbon-carbon bond,is no longer considered synthetically useful, although the aldol reactionthat Wurtz discovered in 1872 has become a staple in organic synthesis. Alexander P. Borodin is also credited with the discovery of the aldol reaction together with Wurtz. In 1872 he announced to the Russian Chemical Society the discovery of a new by-product inaldehyde reactions with properties like that of an alcohol, and he noted similarities with compounds already discussed in publications by Wurtz from the same year. 2. Nielsen, A. T.; Houlihan, W. J. Org. React. 1968, 16, 1-438. (Review). 3. Still, W. C.; McDonald, J. H., III. Tetrahedron Lett. 1980, 21, 1031-1034. 4. Mukaiyama, T. Org. React. 1982, 28, 203-331. (Review). 5. Mukaiyama, T.; Kobayashi, S. Org. React. 1994, 46, 1-103. (Review on tin(II) enolates). 6. Johnson, J. S.; Evans, D. A. Acc. Chem. Res. 2000, 33, 325-335. (Review). 7. Denmark, S. E.; Stavenger, R. A. Acc. Chem. Res. 2000, 33, 432-440. (Review). 8. Yang, Z.; He, Y.; Vourloumis, D.; Vallberg, H.; Nicolaou, K. C. Angew. Chem. Int. Ed. 1997, 36, 166-168. 9. Mahrwald, R. (ed.) Modern Aldol Reactions,Wiley-VCH: Weinheim, Germany, 2004. (Book). 12. Doherty, S.; Knight, J. G.; McRae, A.; Harrington, R. W.; Clegg, W. Eur. J. Org. Chem. 2008,1759-1766. 14. Trost, B. M.; Brindle, C. S. Chem. Soc. Rev. 2010, 39, 1600-1632. (Review). 15. Gazaille, J. A.; Abramite, J. A.; Sammakia, T. Org. Lett. 2012, 14, 178-181. 16. Esumi, T.; Yamamoto, C.; Tsugawa, Y.; Toyota, M.; Asakawa, Y.; Fukuyama Y. Org. Lett. 2013, 15, 1898–1901. 编辑自: 一、Name Reactions (A Collection of Detailed Reaction Mechanisms), Jie Jack Li, Aldol condensation,page 3-5. 二、Strategic Applications of Named Reactions in OrganicSynthesis, László Kürti and Barbara Czakó, aldol reaction, page 8-9. 三、化学空间:https://cn.chem-station.com/reactions/%E5%8A%A0%E6%88%90%E5%8F%8D%E5%BA%94/2014/05/%E4%BA%A4%E5%8F%89%E7%BE%9F%E9%86%9B%E7%BC%A9%E5%90%88%E5%8F%8D%E5%BA%94cross-aldol-reaction.html 相关反应返回搜狐,查看更多 |
【本文地址】
今日新闻 |
推荐新闻 |