Frontiers |
您所在的位置:网站首页 › 塞外江南是指哪两个平原 › Frontiers |
Frontiers
REVIEW article
Front. Sustain., 08 February 2023Sec. Sustainable Chemical Process Design
Volume 4 - 2023 |
https://doi.org/10.3389/frsus.2023.1093911
State-of-the-art review of porous polymer membrane formation characterization—How numerical and experimental approaches dovetail to drive innovation
 Sven Johann Bohr1,2* Sven Johann Bohr1,2*  Fei Wang3* Fei Wang3*  Michael Metze4 Michael Metze4  Josipa Lisičar Vukušić1 Josipa Lisičar Vukušić1  Andreas Sapalidis5 Andreas Sapalidis5  Mathias Ulbricht2 Mathias Ulbricht2  Britta Nestler3,6 Britta Nestler3,6  Stéphan Barbe1*
1Faculty of Applied Natural Sciences, Technische Hochschule (TH) Köln—Cologne University of Applied Sciences, Leverkusen, Germany
2Department of Technical Chemistry II, University of Duisburg-Essen, Essen, Germany
3Institute of Applied Materials-Computational Materials Science (IAM-CMS), Karlsruhe Institute of Technology (KIT), Karlsruhe, Germany
4Sartorius-Stedim Biotech GmbH, Göttingen, Germany
5Institute of Nanoscience and Nanotechnology, National Centre for Scientific Research Demokritos, Agia Paraskevi, Greece
6Institute of Digital Materials Science, Karlsruhe University of Applied Sciences, Karlsruhe, Germany Stéphan Barbe1*
1Faculty of Applied Natural Sciences, Technische Hochschule (TH) Köln—Cologne University of Applied Sciences, Leverkusen, Germany
2Department of Technical Chemistry II, University of Duisburg-Essen, Essen, Germany
3Institute of Applied Materials-Computational Materials Science (IAM-CMS), Karlsruhe Institute of Technology (KIT), Karlsruhe, Germany
4Sartorius-Stedim Biotech GmbH, Göttingen, Germany
5Institute of Nanoscience and Nanotechnology, National Centre for Scientific Research Demokritos, Agia Paraskevi, Greece
6Institute of Digital Materials Science, Karlsruhe University of Applied Sciences, Karlsruhe, Germany
Porous polymer membranes substantially contribute to an acceleration of sustainability transformation based on the energy efficient separation of liquid and gaseous mixtures. This rapid shift toward sustainable industrial processes leads to an increased demand for specifically tailored membranes. In order to predict membrane performance factors like permeability, selectivity and durability, the membrane formation process by film casting and phase inversion needs to be understood further. In recent years, computational models of the membrane formation process have been studied intensely. Their high spatial and temporal resolution allows a detailed quantitative description of phase inversion phenomena. New experimental techniques complement this development, as they provide quantitative data, e.g., on compositional changes of the polymer solution during membrane formation as well as the kinetic progression of the phase separation process. This state-of-the-art review compiles computational and experimental approaches that characterize the phase inversion process. We discuss how this methodological pluralism is necessary for improving the tailoring of membrane parameters, but that it is unlikely to be the way to the ultimate goal of a complete description of the evolution of the membrane structure from the initial demixing to the final solidification. Alternatively, we formulate an approach that includes a database of standardized and harmonized membrane performance data based on previously publicized data, as well as the application of artificial neural networks as a new powerful tool to link membrane production parameters to membrane performance. 1. IntroductionThe increasing demand for innovative high performance porous polymer membranes (hereafter referred to as membranes) surges with the demand for sustainable industrial development. Today, membrane technology has already outcompeted conventional thermal separation processes in a number of industrial sectors, e.g., water desalination. In other applications, such as energy generation, pharmaceutical production, and general separation tasks in the manufacture of chemicals and electronics, membrane processes play an essential role. In membrane technology lies a huge potential for the further optimization of a wide range of chemical and biochemical process operations (Drioli et al., 2011, 2012). Membranes essentially build very thin interfaces allowing selective mass and heat transfer between different phases. Its wide range of applicability is based on the choice of membrane material and its arrangement in space. Therefore, an intimate knowledge of the membrane formation process from start to finish is important for the realization of the potential of membrane technology. In case of porous polymer membranes, this process consists of (1) the preparation of a polymer solution, (2) casting/ shaping into a liquid film or capillary, (3) the initial phase separation, (4) coarsening and structure evolution, (5) solidification of the final morphology, (6) post treatments such as drying, conditioning, winding and storing. Steps 3–5 describe the emergence of the membrane structure. This phase inversion process is realizable with one of the techniques described in Figure 1. The dry phase inversion methods are thermally induced phase separation (TIPS), evaporation induced phase separation (EIPS) and non-solvent vapor induced phase separation (VIPS). Wet phase inversion is called liquid non-solvent induced phase separation (NIPS). The phase inversion process always follows the same physical principles of heat and matter transfer. It consists of multiple non-equilibrium processes that make the prediction of membrane parameters such as porosity and permeability a challenge still to be met. That is why even today, more than a century after the preparation of the first porous polymer membrane (Bechhold, 1907), membrane research is still for the most part an empirical science and lacks a comprehensive theory. FIGURE 1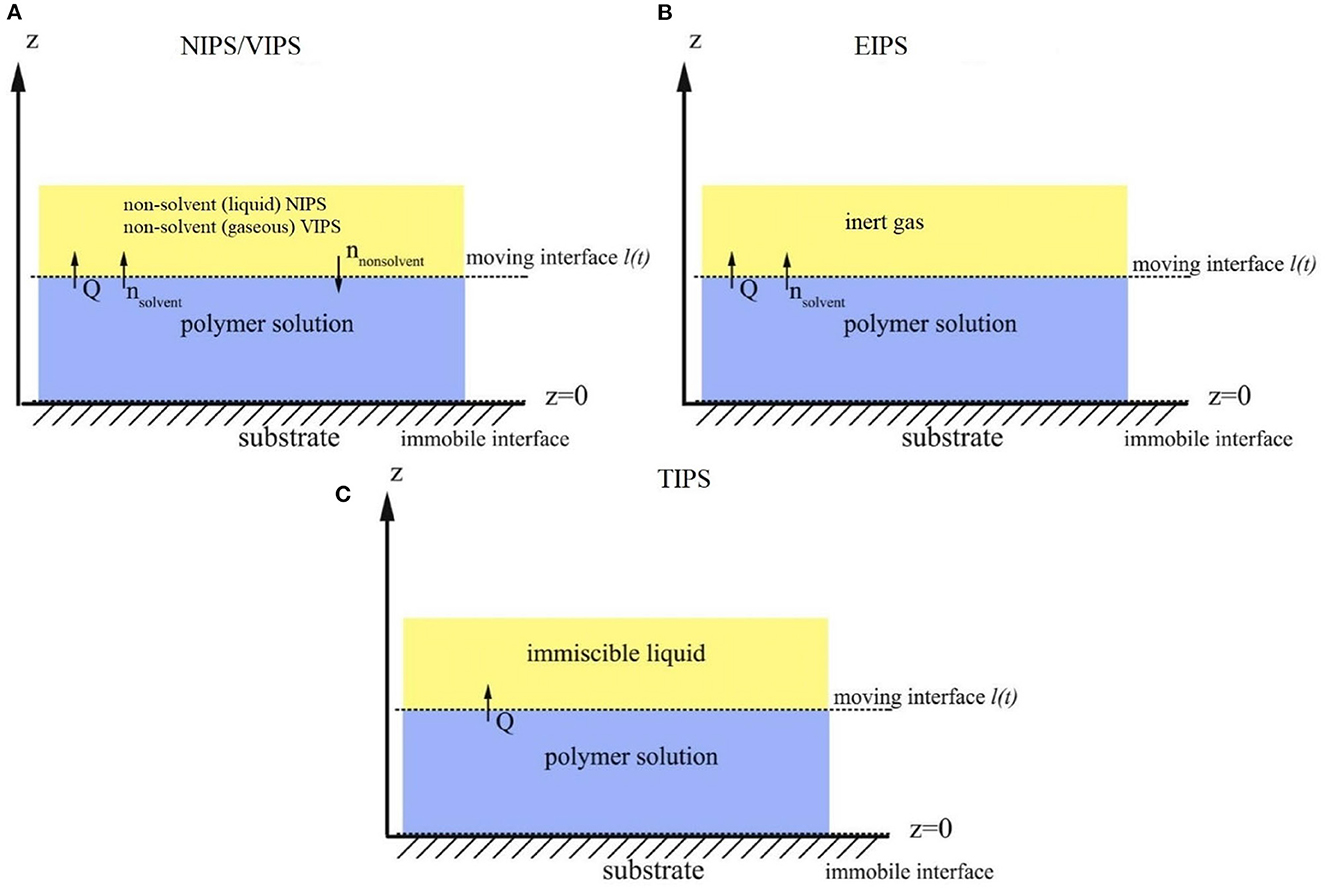 Figure 1. Schematic diagrams of a cross-section of a cast film on a substrate and the relevant physical processes and phenomena during phase inversion. z = 0 represents the dope/support interface and l(t) represents the upper interface. Its location on the z-axis is a function of time due to excess volume of mixing effects as well as solvent and non-solvent diffusion across the interface. (A) NIPS/VIPS is considered an open system. The contact of a cast film with a liquid or gaseous phase of miscible non-solvent results in mass transfer of solvent and non-solvent across the interface as well as heat transfer due to enthalpy of vaporization, or if a temperature gradient between the phases exists. (B) EIPS is also an open system, where the cast film is in contact with an inert gas such as nitrogen, which leads to matter transport out of the film by evaporation, again coupled with heat transfer due to enthalpy of vaporization, or if a temperature gradient between the phases exists. (C) TIPS is a closed system as only heat transfer occurs due to a temperature gradient between cast film and the immiscible liquid phase. Adapted from Tang et al. (2021a) with permission. The membrane formation process can be characterized by experimental and computational approaches. In order to predict structural and mechanical properties of membranes, the membrane formation process needs to be visualized from start to finish. This can only be achieved by superimposing various investigative approaches in an attempt to depict the reality of the membrane formation process in its entirety. Ultimately, membrane properties could be predicted by knowledge of the system composition and the process parameters alone. Although some the experimental approaches were established decades ago, no comprehensive review of methods for the investigation of membrane formation kinetics exists to the best of our knowledge. In comparison, several recent review articles, a book chapter and a perspective article highlight the relevance of modeling various aspects of the formation process of porous polymer membranes at different length and time scales. Some focus on the thermodynamics and kinetics of phase inversion and the resulting morphologies (Müller and Abetz, 2021; Tang et al., 2021a; Wang et al., 2022). Others review the modeling of membrane performance with respect to pore structure and pore size distribution (Mondal et al., 2019), or evaluate future trends in membrane materials and manufacturing techniques to widen the applicability of membrane technology (Nunes et al., 2020). In view of this asymmetric coverage of approaches of membrane formation characterization, the authors of this review put the focus on experimental methods in order to complement the emphasis on modeling approaches apparent in the recent literature. This review describes and evaluates various numerical and experimental methods and for the first time highlights their potential for mutual benefit and possible synergetic effects. The spatial and temporal resolution of the compiled methods will be compared, as well as their possible application during the steps 3–5 of the membrane formation process. In a final analysis, we will discuss the possibility of interlocking computational and experimental approaches in a way that quantitative, experimental data feeds numerical models in order to validate them, and in turn use numerical models to help tailor membrane properties. While the scope here is on porous polymer membranes, analogous activities are taking place in the field of thin film composite membranes via interfacial polymerisation. 2. Methods 2.1. Experimental methods for membrane formation characterizationA brief description of the membrane formation process will help to categorize the different experimental methods. Membrane formation by phase inversion usually starts with a homogenous, thermodynamically stable polymer solution. A new equilibrium point is set inside the miscibility gap of the system by a change in temperature (TIPS) or by bringing the cast polymer solution into contact either with a liquid phase containing a non-solvent (NIPS, VIPS) or with an inert gaseous phase into which the solvent evaporates (EIPS). The thermodynamic state of the system moves toward the new equilibrium point. The system destabilizes progressively until the binodal is crossed (see Figure 2A). At that point, the system becomes metastable and phase separation into a polymer-rich and a polymer-lean phase eventually occurs. If the system crosses the spinodal, the system becomes unstable; polymer-rich and polymer-lean phases separate immediately. Both phases undergo coarsening and the polymer-rich phase eventually solidifies, locking in the intermingled two-phase structure. FIGURE 2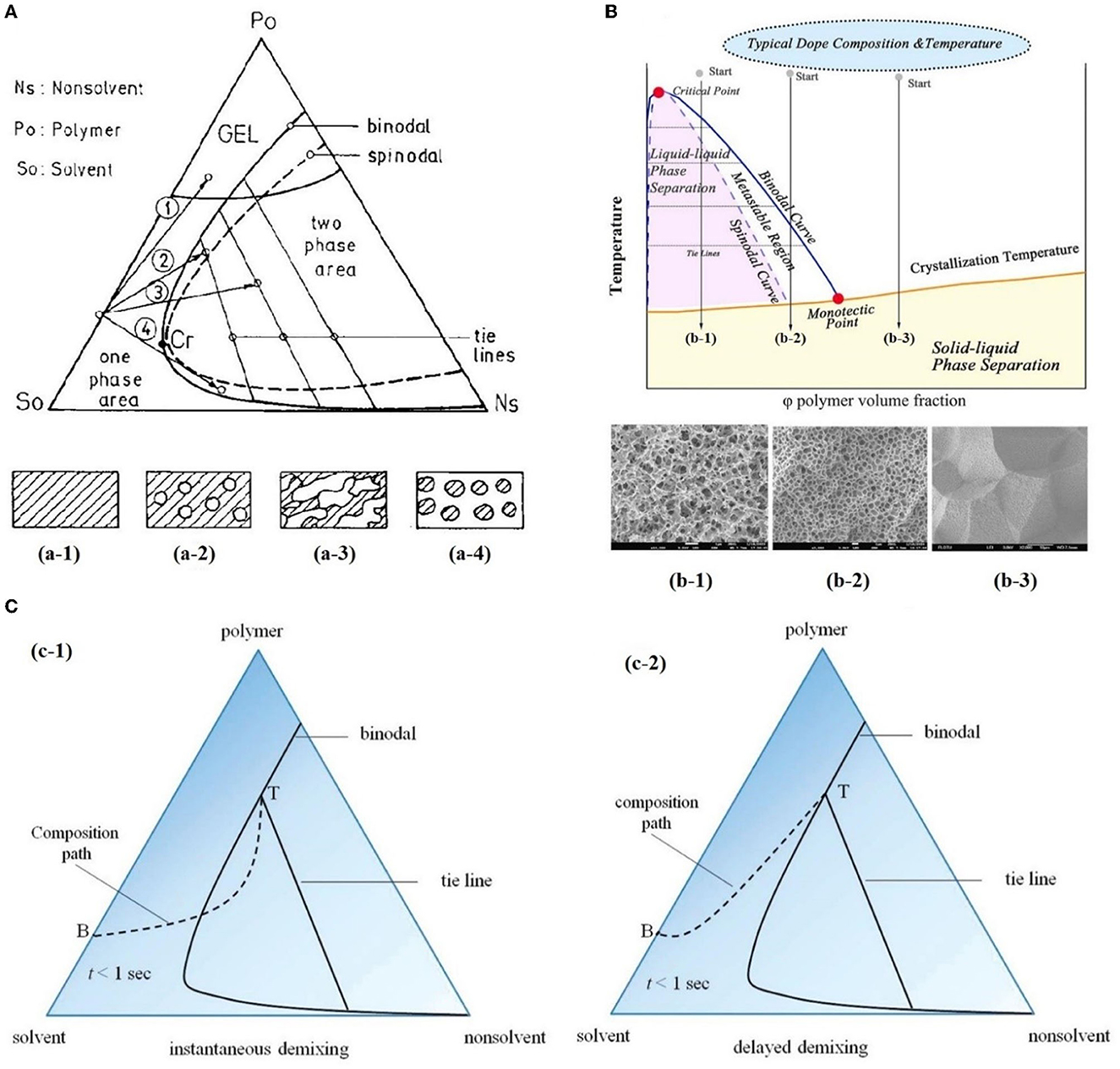 Figure 2. (A) A ternary phase diagram for polymer, solvent and non-solvent with different composition paths that represent possible phase separation mechanisms. (a-1) Solidification, (a-2) nucleation and growth of the polymer-lean phase, (a-3) spinodal decomposition, (a-4) nucleation and growth of the polymer-rich phase. Reproduced from Stropnik et al. (1996) with permission; (B) A temperature-composition phase diagram for a binary solution consisting of polymer and solvent. Thermal quenching of different compositions, and therefore through different regions of the phase diagram, lead to different phase separation mechanisms and subsequently to different membrane morphologies. (b-1) Liquid-liquid phase separation by spinodal decomposition leads to a bi-continuous open-pore structure. (b-2) Liquid-liquid phase separation by nucleation and growth of the polymer-lean phase leads to cellular morphology. (b-3) Solid-liquid phase separation by nucleation and growth of a solid phase leads to semi-crystalline pore structure, whose appearance is governed by the size and connectedness of the solid particles. Adapted from Tang et al. (2021b) with permission; (C) The dashed line shows the composition profile of the film immediately after immersion in the coagulation bath (t < 1 s). T and B represent the compositions at the top and on the bottom of the cast film, respectively. Depending on whether the binodal is crossed immediately or not, two types of demixing can be distinguished resulting in distinct membrane morphologies. (c-1) A fast exchange of solvent and non-solvent initiates instantaneous demixing, resulting in a finger-like structure, prone to macrovoids. (c-2) A slow exchange of solvent and non-solvent initiates delayed demixing, resulting in a sponge-like morphology. Adapted from Guillen et al. (2011) with permission. A specific membrane structure is obtained by conserving a specific non-equilibrium state reached by a specific composition path in a specific time. The basic principles to describe the system and the process are thermodynamics and kinetics, respectively. Therefore, the experimental methods will be divided into thermodynamic and kinetic approaches. The thermodynamic studies focus on the description of the possible states of the polymer liquid mixture, such as phase stability and appearance dependent on composition. The kinetics of membrane formation can be described by two mechanisms, namely diffusion processes and demixing processes. Hence, the kinetic studies are divided further into approaches that either quantitatively measure the change of composition of the polymer solution, or that qualitatively describe the demixing process from a phenomenological point of view. The experimental methods will be discussed regarding their applicability to the membrane manufacturing techniques TIPS, NIPS, VIPS, and EIPS, as well as their aptitude to describe the membrane formation process qualitatively and quantitatively. Section 3.1.4 contains a tabular summary of the experimental methods discussed in the following section. 2.1.1. Thermodynamic characterization of the polymer solution 2.1.1.1. Phase diagramA phase diagram projects all possible thermodynamic states of the investigated system into a Cartesian coordinate system. A phase diagram can be binary, ternary, quaternary, or of even higher orders, depending on the number of system components. Figures 2A, B show a ternary phase diagram and a binary phase diagram, respectively, for a polymer solution. 2.1.1.2. Cloud point determination methodsCorrelations between the thermodynamic state of the polymer liquid system and membrane structure can be investigated by experimental determination of phase diagram elements such as binodal, spinodal, gelation-, crystallization-, vitrification-, and tie-lines. A standard method to characterize a polymer solution thermodynamically is cloud point determination. At the cloud point, the solution turns from clear to turbid. In a phase diagram, the cloud point curve forms the border between solution compositions or temperatures that are thermodynamically stable, and compositions or temperatures that are thermodynamically metastable. Cloud points are measured by titration with a non-solvent and by cooling. The titration method goes back to Schulz (1937) and requires a ternary system consisting of polymer, solvent and non-solvent. Either pure non-solvent (Arahman et al., 2016; Wang et al., 2016; Liu G. et al., 2019; Nivedita et al., 2020) or a mixture of solvent and non-solvent (Yin et al., 2015; Brami et al., 2017; Mazinani et al., 2017; Zhang et al., 2018) is added to a polymer solution at a constant temperature. A mixture of solvent and non-solvent impedes premature coagulation of the polymer at the point of contact with the polymer solution (Reuvers, 1987). Turbidity is detected either visually or by light transmission measurement (Matsuyama et al., 2002b; Schaper, 2002; Zuo, 2006; Rahman et al., 2008; Madaeni and Bakhtiari, 2012; Metze, 2014; Wenning et al., 2018). A second cloud point may be measured by back-titration with solvent after the onset of turbidity. This procedure can be repeated to increase throughput (Mazinani et al., 2017; Yam-Cervantes et al., 2019). If the addition of non-solvent is continued after the onset of turbidity, the solution eventually separates into a polymer-rich and a polymer-lean phase. At this point, the spinodal is crossed (Nivedita et al., 2020). Small angle neutron scattering (SANS) may also be employed to detect phase separation (Metze et al., 2017). The cooling method goes back to Powers (1942) and can be applied to binary and ternary systems. A sample in a sealed glass vial is put in a thermostatic shaking bath. The bath is heated until the sample turns clear. Subsequently, the bath is cooled with a constant cooling rate. At a certain temperature, the solution becomes thermodynamically unstable and demixing occurs (Klein and Schiedermaier, 1970; van Emmerik and Smolders, 1972; Mulder, 1996; Cheng et al., 1999b). Another technique for cloud point determination by cooling employs two microscope cover slips and a spacer of ~100 μm thickness with a center opening. The sample is placed in the opening and is sealed in by the cover slips. The sample is heated on a hot stage and cooled at various constant cooling rates. The onset of turbidity is determined either visually (Matsuyama et al., 2000a, 2002a) or by light transmission measurement (Wijmans et al., 1985; Reuvers et al., 1986). The spinodal curve is determined by quenching the sample to different temperatures below the cloud point. If isolated droplets form gradually, the temperature is considered to be in the metastable region between binodal and spinodal. If an interconnected structure forms immediately, the temperature is thought to be in the unstable region beneath the spinodal (Matsuyama et al., 2002b). The cooling technique also gives information on the demixing mechanism. Liquid-liquid demixing is a very fast process and is independent of the cooling rate, whereas the cooling rate is an important parameter of gelation/vitrification/crystallization. Both phase separation mechanisms can be distinguished by measuring the cloud point curve at different cooling rates. If the cooling curve remains the same among different cooling rates, liquid-liquid demixing occurs; otherwise gelation, vitrification or crystallization is suggested (Mulder, 1996). The abovementioned methods for cloud point determination have certain limitations. They do not give any information on the equilibrium phase compositions that exist inside the miscibility gap, namely the orientation of the tie-lines. Furthermore, the cloud point curve coincides with the binodal only in case of a monodisperse polymer system. Otherwise, the onset of turbidity indicates the precipitation of the heaviest polymer fraction. In addition, polymer concentrations beyond the critical point are tedious to assess, because of increasing viscosity. This retards the adjustment of the thermodynamic equilibrium and instead of the true cloud point, a premature coagulation is observed. In case of a ternary system, a linearized cloud point curve correlation can be applied, to extrapolate the course of the cloud point curve to higher polymer concentrations. The correlation is only valid under the assumptions that the polymer is strongly incompatible with the non-solvent and that the phase separation mechanism is liquid-liquid demixing. However, the extrapolation loses accuracy with increasing polymer concentration (Lau et al., 1991; Boom et al., 1993; Law and Mukhopadhyay, 1997; Liu L. Q. et al., 2013; Dong et al., 2014; Liu Y. et al., 2019). These limitations may be overcome with the methods proposed by Kahrs et al. (2019) and Barth and Wolf (2000). They provide experimental data on the course of the cloud-point curve and on the orientation of the tie-lines, even at high polymer concentrations. The methods can be applied to ternary systems consisting of polymer, solvent, and non-solvent. Figure 3 describes the procedure by Kahrs, exemplary. FIGURE 3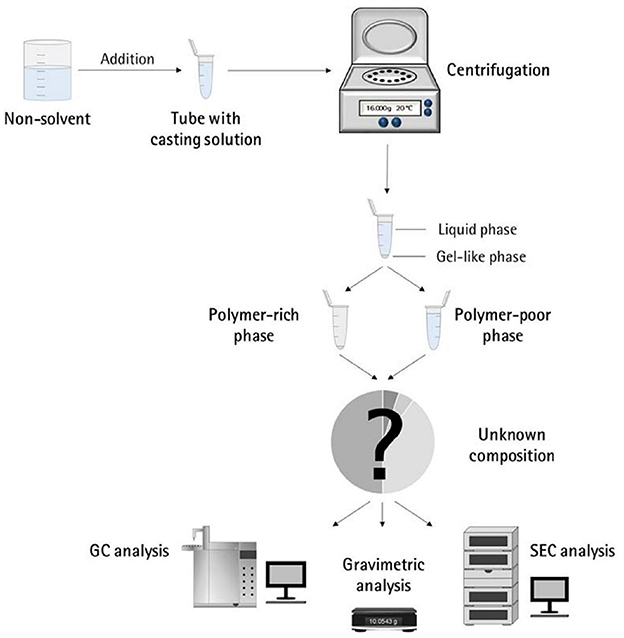 Figure 3. Determination of the cloud-point curve and the tie-lines at high polymer concentrations: Samples with different ratios of polymer solution and non-solvent are prepared. The amount of non-solvent has to be sufficient to initiate phase separation. The samples are homogenized and centrifuged for 24 h in a tempered centrifuge. The polymer-rich phase aggregates at the bottom and the polymer-lean phase floats on top. The polymer content of the respective phase is determined either gravimetrically via evaporation and drying or by size exclusion chromatography (SEC). SEC additionally shows the molecular weight distribution of the polymer. The solvent and non-solvent contents are determined by gas chromatography. Reproduced from Kahrs et al. (2019) with permission. 2.1.1.3. Rheological characterization of polymer solutionsThe rheological characterization of a polymer solution gives information on the solutions flow properties at a given temperature. Polymer solutions with the same amount of the same polymer show different viscosities for different solvents, depending on the solvent quality. Decreasing solvent quality increases the miscibility gap and the viscosity of the polymer solution (Hoernschemeyer, 1974; Kahrs et al., 2020). Furthermore, poorer solvents amplify the effects of additives such as PEG and PVP (Kahrs and Schwellenbach, 2020). Besides the thermodynamic properties of a given polymer/solvent/non-solvent system, the kinetics of the phase separation process also have an influence on the membrane structure and properties. The diffusion rates of solvent and non-solvent are determined by the gradient of chemical potential across the film/bath interface. The diffusion rates are limited by the viscous hindrance of the polymer solution (Lee H. J. et al., 2004; Li et al., 2008; Guillen et al., 2013). The appearance of macrovoids seems to be governed by the rate of non-solvent inflow and polymer chain entanglement (Lin et al., 2002; Ren et al., 2010; Wang and Ma, 2012; Hung et al., 2016). Cloud point experiments may show a decreasing thermodynamic stability with increasing polymer concentration, suggesting a faster phase separation. However, coincidentally the viscosity of the polymer solution increases. This in turn leads to a decrease of the diffusion rate during phase inversion, resulting in a slower phase separation (Lee H. J. et al., 2004). It depends on the specifics of the polymer/solvent/non-solvent system whether the thermodynamic instability or the viscosity hindrance controls the phase separation (Zhang et al., 2010; Sadrzadeh and Bhattacharjee, 2013; Venault et al., 2016). The phenomenological model, Pη0-1, predicts porosity and membrane thickness. The first derivative of the Gibbs free energy of mixing, P, performs as the thermodynamic driving force of non-solvent diffusion, while the zero shear viscosity of the polymer solution, η0, behaves as a resistive force. The former may be calculated by the compressible solution model of Mayes (Ruzette and Mayes, 2001; Gonzalez-Leon and Mayes, 2003), while the latter may be measured experimentally (Bazarjani et al., 2009; Ghasemi and Mohammadi, 2013). A polymer solution that undergoes phase separation decomposes into two thermodynamically stable solution that coexist next to each other. The rheological properties of the phases differ significantly. The polymer-rich phase has a comparatively high viscosity due to high polymer content. This may lead to chain entanglement as well as inter- and intramolecular hydrogen bridges, which form a network-like superstructure that surrounds the macromolecules like a fog (Peng et al., 2009; Mezger, 2014). Thus, the polymer-rich phase also shows elastic properties. In contrast, the polymer-lean phase is mostly made up of small solvent and non-solvent molecules and exhibits low viscosity and no elasticity. Due to the distinct flow properties of the two phases, the mixture is called dynamically asymmetric. Tanaka's model of viscoelastic phase separation describes how a porous membrane structure arises from this asymmetry. The gel-like polymer-rich phase exhibits a long relaxation time and cannot catch up with the phase separation itself. A network-like or sponge-like structure of polymer-rich phase emerges. Its volume shrinks with time due to out-diffusion of solvent. The shape of the polymer-rich domains is determined by elastic forces and not by interfacial tension (Tanaka, 1996, 1997, 2000). Increased viscoelasticity promotes the preservation of the nascent lacy structure with interconnected pores (Tsai et al., 2010; Mousavi and Zadhoush, 2017). The structure evolution eventually comes to a halt due to solidification of the polymer-rich phase. 2.1.2. Process kinetics of phase inversion characterized by visualizing the demixing processThe experimental methods discussed in this section phenomenologically describe the membrane formation with a microscope to yield qualitative and quantitative information on the phase inversion process. 2.1.2.1. Optical methods 2.1.2.1.1. Optical microscopyOptical methods visualize the membrane forming process unmediated. They use light of the entire visible spectrum to gather data. Qualitative aspects of the membrane forming process, such as the formation of droplets, the appearance of the emerging membrane structure, or the movement of the coagulation front can be seen. These phenomenological observations yield quantitative parameters like droplet growth rate and apparent diffusion coefficient. The experimental setup and the sample preparation for the optical microscopy (OM) technique are as follows. A sample of the polymer solution is placed on or between microscope slides, which are in turn placed on a heating stage in the optical path of a microscope. The observation is carried out under isothermal conditions, or with a constant cooling rate. To increase the contrast, the sample is dyed, e.g., with Rhodamine B or phenolphthalein. The dying of the sample is avoided by using a phase contrast microscope (Lee H. J. et al., 2004). During TIPS, OM determines the initial phase separation mechanism, namely nucleation and growth or spinodal decomposition. In case of initial spinodal decomposition, the interconnected structure often coalesces into droplets that over time grow and decrease in number. A quench path below the critical point exhibits the growth of polymer-rich droplets in a polymer-lean matrix. Here, the droplet growth rate increases with decreasing quench temperature and decreasing polymer concentration. In comparison, a quench path above the critical point leads to the growth of polymer-lean droplets in a polymer-rich matrix. This quench path shows the same growth rate dependence on quench temperature, but the opposite dependence on polymer concentration (Smolders et al., 1971; McGuire et al., 1995). A respective binary phase diagram is shown in Figure 2B. OM is capable of distinguishing between liquid-liquid demixing and solid-liquid demixing during TIPS (Yoo and Kim, 2008; Yang et al., 2013; Ishigami et al., 2014). OM also shows the impact of polymer molecular weight on droplet growth rate and membrane morphology. With a high molecular weight polymer, the droplets grow to a constant diameter before the polymer-rich phase solidifies. This leads to a membrane structure with small, interconnected pores. For a low molecular weight polymer, the growth period is shorter, but the growth rate is higher, which leads to bigger droplets overall. This is attributed to the lower viscosity of the polymer-rich phase. The membrane structure in this case is of large cellular pores. The addition of a low molecular mass co-polymer decreases the droplet size with increasing amount, resulting in smaller pores (Matsuyama et al., 2002a; Yave et al., 2005). If OM is applied to NIPS, the movement of the coagulation front with time and the qualitative distinction between sponge-like and finger-like structure growth is observed (Matz, 1972). The movement follows Fick's first or second law of diffusion, depending on the investigated polymer system and on the properties of the non-solvent. The diffusivity decreases with increasingly softer non-solvents. That leads to a denser membrane with smaller pores and thicker skin, whereas high diffusivity promotes the growth of finger-like structures and high porosity membranes (Wang et al., 1998; Fan et al., 2002). Another description of the coagulation front suggests three distinct linear correlations for its movement. They correspond to three consecutive gelation steps. First, the formation of a skin layer, second the formation of a transition layer and third the formation of a sub layer. These layers can be distinguished in the cross-section of the final membrane. It was found that low molecular weight polymer additives increase the gelation rate with increasing concentration, whereas a non-ionic surfactant does so only up to a certain concentration, at which the effect reverses (Qin et al., 2006a,b; Zhao et al., 2011; Fang et al., 2015). OM observations suggest that macrovoids emerge because of convective inflow of non-solvent into the polymer solution. The formation of a skin layer or a highly viscous gel layer at the air-side interface hinders macrovoid formation only if the hindrance exceeds the convective inflow on non-solvent (Guillen et al., 2013). A humidity-controlled glove box allows the investigation of VIPS processes with OM. This setup showed an increase in pore size with an increase of relative humidity and a decrease in polymer concentration, due to coarsening of the polymer lean-phase by coalescence (Chae Park et al., 1999). It also demonstrated the occurrence of spinodal decomposition at the early stage of the membrane formation and the subsequent formation of a liquid surface layer on top of the air-side interface (Lee H. J. et al., 2004; Menut et al., 2008). A hydrophilic co-polymer increases non-solvent diffusivity. OM analysis suggests this is due to the enhanced hydrophilicity of the polymer solution, rather than the decrease of polymer solution viscosity (Venault et al., 2016). With OM, phase separation phenomena, such as the rate of structure formation and its appearance are directly observable. Thereby, instantaneous and delayed demixing are distinguished and whether the membrane morphology is going to be sponge-like or finger-like may be predicted up to a certain point. The effects of additives, solvents and non-solvents, as well as the effects of variations of process parameters on the kinetic progression of the phase inversion process can also be investigated. However, OM gives only limited information on the behavior of the non-solvent and no information on the behavior of the solvent. A further limitation is the sample preparation. The sample is placed in the gap between two microscope slides. The ratio of air-side interface area to the polymer solution thickness is much smaller than during industrial scale or lab-scale membrane preparation. 2.1.2.1.2. Confocal laser scanning microscopyAnother optical method uses confocal laser scanning microscopy (CLSM) to study in-situ and in real time the mechanism and the kinetics of structure formation during EIPS. A closed measurement cell with inlet and outlet facilitates the controlled evaporation of the solvent from the cast polymer film by perfusion of a defined gas flow (Metze et al., n.d.). Figure 4 shows the results of model cases for demixing by spinodal decomposition and by nucleation and growth. FIGURE 4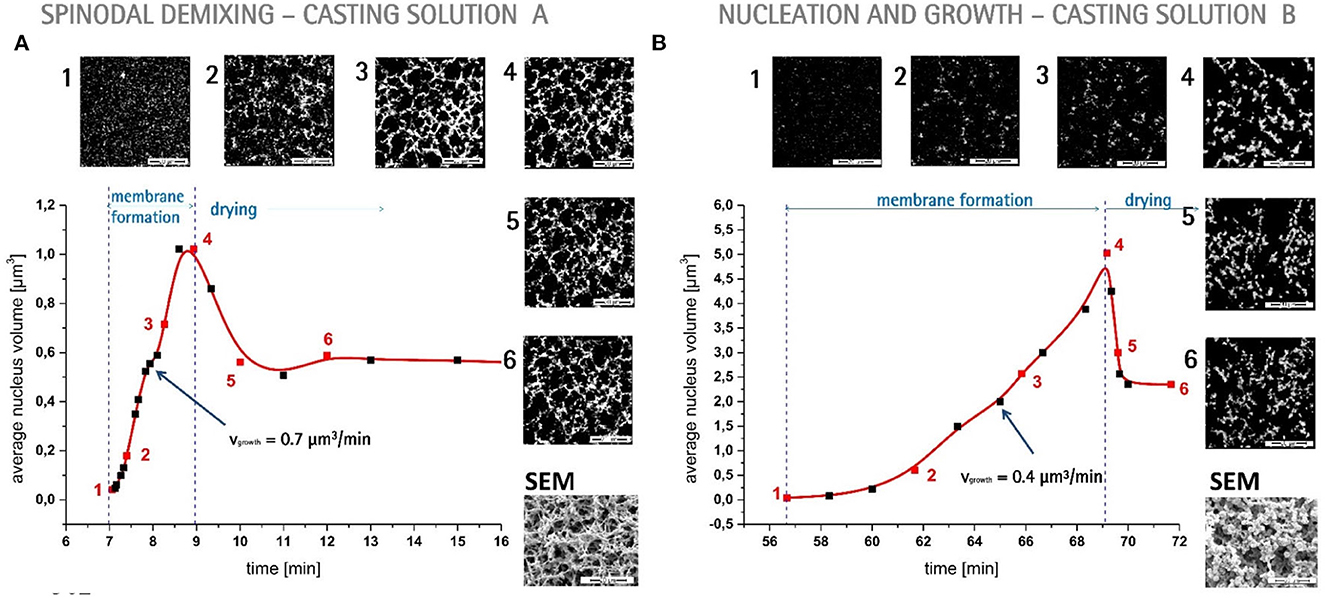 Figure 4. (A) The composition path crosses the binodal near the critical point, inducing spinodal decomposition. Membrane formation starts after 7 min and is finished after 2 min. (B) The initial composition of the casting solution contains less polymer. The composition path crosses the binodal below the critical point inducing nucleation and growth of polymer-rich particles in a polymer-lean matrix. Membrane formation starts after 1 h and lasts for 10 min. (A, B) bottom right: SEM images of the final membrane structures. Adapted from Metze et al. (n.d.) with permission. 2.1.2.1.3. Optical coherence tomographyOptical coherence tomography (OCT) is an emerging technique for non-invasive 3D imaging of scattering matter, similar to CLSM. OCT exploits low coherence interference of visual light for its depth resolution. It gathers intensity data at various depths simultaneously and thereby achieves a higher scan rate than CLSM. It is used for the direct observation of fouling, scaling and wetting phenomena of membranes (Liu et al., 2020, 2021; Shao et al., 2022). OCT applied to the NIPS process enables the measurement of the movement of the coagulation front as a function of time, similar to OM (Tu et al., 2021). The main advantage of OCT over OM is the ability of in-situ measurement of non-solvent indiffusion with an experimental environment similar to common membrane preparation techniques. 2.1.2.2. Light scattering methods 2.1.2.2.1. Light transmission methodLight scattering methods are transmission measurements that utilize light sources either of the entire visible spectrum or of a certain wavelength. After passing the sample, the remaining light is gathered by a photo detector that emits an electric current proportional to the transmitted light intensity. The light transmission (LT) technique was established by Reuvers (1987) in order to differentiate between instantaneous and delayed liquid-liquid demixing and to correlate the results with the emergence of finger-like and sponge-like membrane morphologies, respectively. Both demixing mechanisms are shown in Figures 2C c-1, c-2, respectively. LT tracks the progression of the membrane formation process by measuring the increasing turbidity of the polymer solution. The turbidity is due to the difference of refractive indices of the demixed phases. Figure 5 shows the experimental setup. FIGURE 5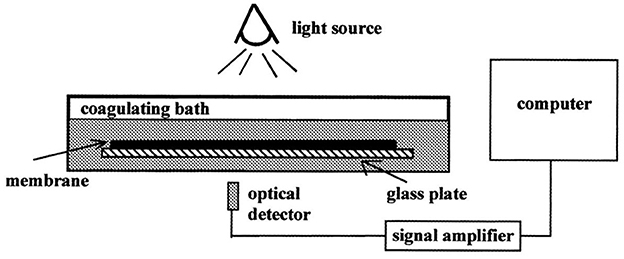 Figure 5. The LT experimental setup consists of a light source that shines a collimated light beam through a cast film that has been applied to a transparent support into a photo sensor. The light intensity is recorded as a function of time. The setup is complemented by a coagulation bath, a hot stage or a glove box with controlled airflow and humidity, depending on the investigated phase separation process. Reproduced from Machado et al. (1999) with permission. By analyzing the plot of light intensity as a function of time, derived from a LT experiment, two parameters β and Imin can be defined. β = −dI/dt reflects the rate of solidification of the membrane. Large β corresponds to a fast fixation of a looser membrane structure, whereas small β signifies slow gelation and a denser membrane structure. Imin = I/I0 = const is defined as the light transition intensity when β = 0. It reflects the density of the membrane and a denser membrane has a higher Imin value. On a side note, large macrovoids also increase the Imin value. With these parameters, Reuvers' theory of instantaneous and delayed demixing and their implications on membrane structure is complemented with a semi-quantitative analytical method (Hao and Wang, 2003). In case of a volatile solvent, the membrane formation during TIPS is initiated by a change of temperature or a change of concentration due to solvent evaporation, since both factors change the thermodynamic stability of the polymer solution. A comparison of LT studies of a closed and an open system differentiate the effects of said factors on the membrane morphology. IN this case, the system boundaries are the confines of the polymer solution. A closed system eliminates evaporation as a means of membrane formation mechanism so only the change in temperature has an effect. An open system under isothermal conditions allows the investigation of the role evaporation plays during the phase inversion process. With this approach, phase separation mechanisms such as liquid-liquid demixing and solid-liquid demixing, as well as various solidification mechanisms such as gelation and vitrification or crystallization can be investigated, and a wide range of membrane morphologies is obtained (Luo et al., 2003). During NIPS, LT experiments show the effects of changes of composition on precipitation kinetics. An increasing solvent content in the non-solvent bath shows a decreasing precipitation rate associated with a dense, sponge-like morphology, whereas a decrease of polymer content in the polymer solution leads to an increasing precipitation rate associated with a finger-like structure and the possibility of macrovoid formation (Madaeni and Bakhtiari, 2012). In another case, the increase of the solvent ratio in the coagulation bath led to the observation of an oscillatory behavior of precipitation kinetics and macrovoid formation. A qualitative model was proposed that considered three distinct layers in the cast film, each with different mass transfer resistances controlling the precipitation rates of the respective layer and thereby the morphology (Machado et al., 1999). Non-solvents may be distinguished by their ability to promote phase separation. In a given polymer/solvent system a hard non-solvent presents a large miscibility gap and generally promotes instantaneous demixing, which is associated with fast liquid-liquid phase separation and at least initial spinodal decomposition. By employing increasingly softer non-solvents, the miscibility gap shrinks and LT experiments illustrate a change from instantaneous to delayed demixing, which is associated with a change of morphology from finger-like to sponge-like, as well as the suppression of macrovoid formation (Fan et al., 2002). A combination of a crystalline polymer and a soft non-solvent shows delayed demixing that is associated with slow nucleation and growth and solid-liquid demixing (Cheng et al., 2001; Zuo, 2006). The quality of a solvent correlates with the viscosity of the polymer solution. A good solvent yields a low viscosity polymer solution and vice versa, given that all other system parameters remain constant. If a good and a bad solvent for a given polymer/non-solvent system show similar precipitation rates but lead to different membrane structures, this can be attributed to differences in the respective phase diagrams and associated thermodynamic properties. A bad solvent shows a wide miscibility gap and a gelation point close to the binodal. This leads to a high viscosity, low mobility polymer-rich phase after the initial demixing. That hinders the further progression of the phase separation process. Thus, the growth of the polymer-lean phase stops early and an isotropic dense membrane structure remains. A good solvent on the other hand shows a comparatively narrow miscibility gap and a gelation point further away from the binodal, which leads to an increased time between onset of turbidity and fixation of membrane morphology by crossing of the gelation point (Kim et al., 2001). Additives, such as water-soluble polymers and nanomaterials both show an enhanced descending rate β during LT experiments with increasing content. Both additives reduce the thermodynamic stability of the system and increase its viscosity. In this case, the decrease in thermodynamic stability outweighs the rheologic hindrance, which leads to a faster phase inversion (Zhao et al., 2011). The addition of a low molecular weight alcohol as a non-solvent additive (NSA) to a polymer solution leads to the opposite effect. Here, the reduced thermodynamic stability of the system outweighs the increase of polymer solution viscosity, resulting in a decreasing precipitation rate with increasing NSA content (Li et al., 2008). During VIPS, LT experiments indicate that an increase of polymer chain length accelerates the demixing process by switching from a slower nucleation and growth mechanism to a faster spinodal decomposition process. Compared to NIPS, the phase inversion is about ten times slower (Zeman and Fraser, 1993, 1994; Li et al., 2008). In summary, LT experiments can distinguish between instantaneous and delayed demixing. In case of a semi-/crystalline polymer, it distinguishes liquid-liquid from solid-liquid demixing. The demixing mechanism influences the membrane morphology. As a rule of thumb, the faster the demixing the more porous the membrane becomes. In combination with thermodynamic data of the investigated system, LT reveals if the phase separation process is controlled thermodynamically or kinetically. Furthermore, it shows the influence of additives on precipitation kinetics. A limitation of the technique is its purely qualitative description of the phase inversion process. This limitation is overcome by analyzing the LT plot and introducing semi-quantitative parameters. 2.1.2.2.2. Small angle light scattering methodThe small angle light scattering (SALS) technique is similar to the LT approach. Additionally, SALS determines the dependence of the scattered light intensity I from the scattering angle θ or the corresponding wavenumber q. Figure 6 shows a schematic representation of a SALS experimental setup. By analyzing the plot of I against θ or q, the initial phase separation mechanism can be determined. FIGURE 6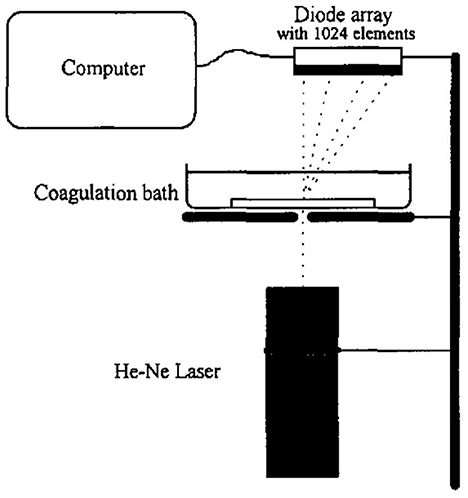 Figure 6. The SALS experimental setup consists of a collimated monochromatic light source, e.g., a He-Ne laser, a transparent casting support and a photo sensor array. The laser beam is applied perpendicular to the cast film and shines through it. The detector gathers the scattered light intensity as a function of the scattering angle and time. Reproduced from Nunes and Inoue (1996) with permission. A monotonic decrease of the scattered light intensity is associated with a nucleation and growth mechanism. At a fixed angle or wavenumber, an increase of scattered light intensity with time is fitted to the following power law, where K is the growth constant, t is time, and τ is the time at which phase separation sets in. I=K(t-τ )n (1)If a plot of logI vs. logt gives a straight line, the slope supplies a value for n. A slope close to 3 indicates heterogeneous nucleation, whereas a slope close to 4 indicates homogenous nucleation. Other values for n are inconclusive. According to linear Cahn theory, a maximum in the I against θ or q plot is associated with a spinodal decomposition mechanism. The angle or wavenumber of maximum scattered light intensity, can be directly correlated to the interphase periodic distance D of the bi-continuous structure observed during spinodal decomposition by the relationship. D= 2πqmax (2)Another requirement for spinodal decomposition, according to linear Cahn theory, is an initial exponential growth of the scattered light intensity with time at a fixed angle. The slope of the plot of ln I vs. time gives R(q), the amplitude growth rate of fluctuations with wavenumber q. R(q) depends on the thermodynamics as well as on the mobility of the system. Still, according to linear Cahn theory, if the relationship of R(q)q2 vs. q2 is linear, then extrapolation to q = 0 gives an estimation of the apparent diffusion coefficient Dapp. Dapp depends both on thermodynamic and kinetic aspects of the system during phase separation (Inoue and Ougizawa, 1989; Nunes and Inoue, 1996). SALS furthermore is a method capable of monitoring the morphological evolution of nascent membranes during later stages of the phase inversion process. Even if liquid-liquid demixing is initiated by spinodal decomposition, phase coarsening via coalescence or Ostwald ripening during later stages of phase separation may lead to a dispersed phase/matrix structure. This results in a final membrane morphology that lacks the high pore interconnectivity usually associated with spinodal decomposition (van Aartsen and Smolders, 1970). In case of ongoing spinodal decomposition during late stage coarsening, the position of the intensity maximum obeys the growth law. qmax∝t-α (3)where α is the growth exponent. A combination with Equation (2) implies that the cessation of domain growth affects the time progression of the scattering maximum. That enables the monitoring of the time and temperature dependence of the size scale of the bi-continuous structure and the arrest of growth due to solidification (Graham et al., 1997). Thus, the kinetics of liquid-liquid phase separation and crystallization can be quantified; insofar that thermal quenching above the crystallization temperature shows increasing domain growth with increasing quench depth and decreasing polymer concentration. Quenching below the crystallization temperature shows only brief coarsening before structure growth stops (Graham and McHugh, 1998). SALS also supplies direct experimental evidence of the initial phase separation mechanism dependent on the polymer concentration. In case of a cellulose acetate/acetone/water system during NIPS, casting solutions with 11–17 wt.% cellulose acetate show initial spinodal decomposition, whereas nucleation and growth is found for casting solutions with cellulose acetate concentrations < 6 and > 25 wt.%. However, a comparison of the final membrane morphologies suggests a transition from the initial bi-continuous structure to a discontinuous morphology with spherical domains, before the system solidifies (Nunes and Inoue, 1996; Zoppi et al., 1999). Furthermore, SALS supplies experimental evidence to theoretical ternary phase diagrams, by identifying the initial phase separation mechanism. The magnitude of Dapp and K reflects the phase separation rate during spinodal decomposition and nucleation and growth, respectively, and allows a qualitative assessment of the relative quench depth at which the phase separation takes place inside the miscibility gap (Barth et al., 2000; Schuhmacher et al., 2001). To sum up, SALS reliably distinguishes spinodal decomposition from nucleation and growth. However, this observation only applies to the skin of the polymer film. Deeper layers of the film reach the thermodynamic conditions for demixing eventually, but enter the miscibility gap at different points. Therefore, different mechanisms of phase separation take place in different layers of the film. In addition, the initial membrane morphology evolves, depending on the mobility of the polymer-rich phase. The kinetics of annealing processes are described by coarsening and Ostwald ripening. As with LT, the SALS approach yields semi-quantitative kinetic parameters that describe the kinetic progression of the phase inversion. Both techniques cannot quantify any change of composition associated with this kinetic progression. 2.1.2.3. Cryo-SEMScanning electron microscopy (SEM) is a standard method to examine a membrane's final morphology. The method can also be used to investigate the structure evolution during phase separation. Snapshots of the nascent membrane's cross-section are made, by taking samples at different times after the initiation of phase separation. The samples are quenched in ice water, liquid ethane or liquid nitrogen. Due to the temperature gradient during sample preparation a shrinkage and possible deformation of the membrane structure is to be expected (Tsai and Torkelson, 1990; Zeman and Fraser, 1994; Graham et al., 1997; Graham and McHugh, 1998; Prakash et al., 2006; Kuo et al., 2008; Nakao et al., 2021). 2.1.3. Process kinetics of phase inversion characterized by measuring the change of compositionAs mentioned in Section 2.1, moving the equilibrium state of the system out of the homogenous region initiates the membrane formation process by phase separation, as soon as the binodal is crossed. The phase separation process eventually comes to a halt due to an increase in viscosity of the polymer-rich phase, freezing the evolving structure in time and space. The composition path describes this progression. The composition path describes the change of composition of a particular plane inside the cast film with time (Su et al., 2017). The investigation of the timely progression of the composition path leads to correlations between the composition of the polymer solution and the process parameters on the one hand and the membrane morphology on the other hand. 2.1.3.1. Gravimetric methodDuring EIPS, the rate of composition change can be measured gravimetrically if the system consists of a polymer and a volatile solvent only, or additional components are considered non-volatile (Zeman and Fraser, 1994; Zhao et al., 2011; Sun et al., 2013). In order to describe the evaporation rate quantitatively the empirical equation. W0-WtW0-W∞=1-exp(-btm ) (4)was proposed by Hung and Feng. W is the weight of the cast film and the subscripts 0, t and ∞ signify the weight before, during and after the EIPS process, respectively. b and m are empirical parameters characterizing the solvent evaporation rate. Rearranging Equation (4) suggests a linear relationship. log[-ln(Wt-W∞W0-W∞)]=mlogt+logb (5)between the left side of Equation (5) and logt with a slope of m and an intercept of logb. Both the values of m and b tend to increase with an increase of evaporation temperature and/or a decrease of film thickness (Huang and Feng, 1995). During VIPS, a normalized volatile molecules ratio Ns in gram of volatile molecules (i.e. solvent and non-solvent) per gram of polymer may be introduced. Ns shows distinct behaviors with time, depending on the relative humidity. Under dry conditions, a monotonic decrease of Ns signifies an expected EIPS characteristic of the system. In a humid atmosphere an initial increase of Ns followed by a decrease confirms emergence of a liquid layer of volatile components on top of the cast polymer solution (Menut et al., 2002; Caquineau et al., 2003; Tsai et al., 2006; Sun et al., 2013). A limitation of this technique is its lack of spatial resolution, as no concentration gradients along the cross-section of the cast film can be resolved. Furthermore, a coagulation path cannot be determined, because different volatile species cannot the distinguished since only the nett change of mass is detected. 2.1.3.2. Spectroscopic methods 2.1.3.2.1. Fourier transform infrared microscopyIt is a challenging endeavor to determine local compositions inside the film experimentally, because the change of composition occurs very fast (often < 1 s) and the film is rather thin (often < 200 μm) (Mulder, 1996). Light scattering techniques such as infrared spectroscopy and Raman spectroscopy characterize the composition path, even under those conditions. As mentioned in Section 2.1.2.1, researchers developed the OM technique to observe the movement of the coagulation front and the formation of the membrane during NIPS (Matz, 1972). With this technique, the inflow of non-solvent can be measured qualitatively. The outflow of solvent cannot be observed. These limitations are overcome by Fourier transform infrared microscopy (FTIRM). FTIRM is a transmission sampling method with a focus area of 20 × 300 μm. This size is necessary to get enough energy intensity for analysis (Sammon et al., 2002). Figure 7 shows the sample preparation and measuring procedure. FIGURE 7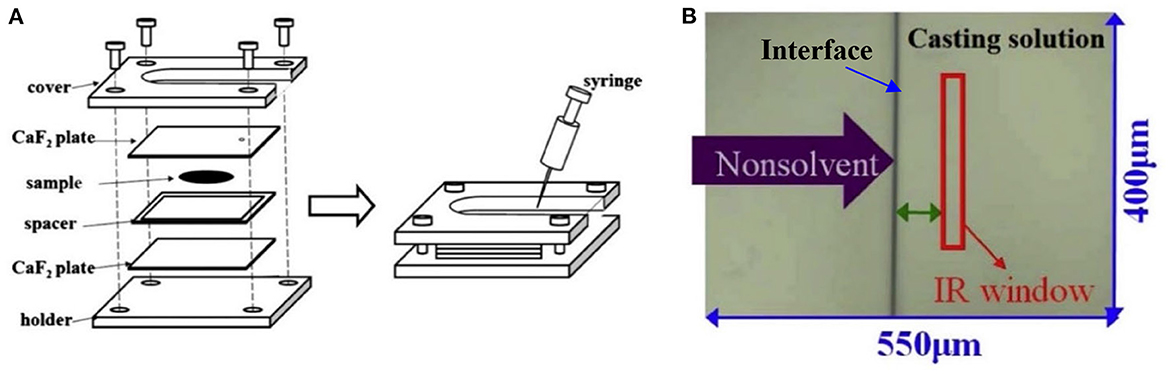 Figure 7. (A) Schematic representation of an FTIRM measurement cell. The sample is put in the gap between two CaF2 plates that are separated by a spacer of 15 μm thickness to ensure transmission of the probing radiation. Reproduced from Kuo et al. (2008) with permission. (B) Sight from above through the measurement cell with the non-solvent approaching from the left side, the casting solution on the right side, the vertical phase interface in the middle and the focus area in red. Reproduced from Su et al. (2017) with permission. The method may also be applied during VIPS by introducing humid air instead of a liquid non-solvent into the gap (Tsai et al., 2010). During NIPS and VIPS, FTIRM measures the time dependent change of composition of any dope component, such as polymer, co-polymer, additive, solvent, co-solvent and non-solvent simultaneously. The focus area may be on either side of the air-side interface, or any plane within the polymer solution. The temporal resolution is 1.1 s. The method complements the phenomenological observations made with OM, e.g. movement of the coagulation front, with information on the composition of the film and its change with time. Lin et al. (2002) showed that the gelation of the film prior to immersion into the non-solvent bath significantly lowers the penetration rate of the non-solvent from seconds to almost 20 min, thus suppressing the formation of macrovoids. The addition of a co-solvent decreases the exchange rate of solvent and non-solvent by forming a barrier that inhibits the indiffusion of non-solvent. A higher outflow rate of the co-solvent compared to the solvent leads to skin formation, due to an increase of polymer concentration at the air-side interface. This decreases the exchange rate of solvent and non-solvent even further und suppresses the formation of macrovoids (Tsai et al., 2002). In contrast, Kuo et al. (2008) showed how a decrease of polymer concentration near the air-side interface due to a solvent/non-solvent exchange ratio < 1 leads to the emergence of a bi-continuous structure that evolves into a nodular structure during late stage coarsening. Furthermore, the time the composition of the cast film remains in the metastable region between binodal and spinodal has an influence on the membrane structure. A short stay in the metastable region promotes spinodal decomposition, whereas if the stay in the metastable region is long enough to initiate nucleation, the structure changes from bi-continuous to cellular (Su et al., 2017). This observation also applies to VIPS, as a shorter stay of the composition path in the metastable region is linked to a higher probability of spinodal decomposition (Su et al., 2009). The investigation of the composition path in addition to the analysis of the composition of the polymer-rich and polymer-lean phase determines whether phase separation occurs within the metastable region or by crossing the spinodal (Tsai et al., 2010). Furthermore, FTIRM documented an emerging liquid surface layer during VIPS. A mechanism that describes the implications of said liquid layer on the morphology of the membrane was proposed my Menut et al. (2008). The FTIRM technique has certain limitations, such as the rapid saturation of the coagulation bath or vapor cloud due to the small volume of the liquid cell (Karimi and Kish, 2009). Consequently, the common boundary condition of constant concentration inside the coagulation bath or vapor cloud does not hold. Due to FTIRM being a transmission sampling method, the interface between coagulation medium and polymer solution is very thin (~15 μm). The experimental setup therefore does not adequately represent the interface conditions of an industrial scale, or even a lab scale membrane production environment. Finally, the boundary effect of the casting support is not considered during FTIRM (Tsai et al., 2010). 2.1.3.2.2. Attenuated total reflectance-fourier transform infrared spectroscopyThe aforementioned limitations of FTIRM are overcome by attenuated total reflectance-fourier transform infrared spectroscopy (ATR-FTIR) spectroscopy. ATR-FTIR is a contact sampling method with limited penetration depth. Figure 8 shows the experimental setup. FIGURE 8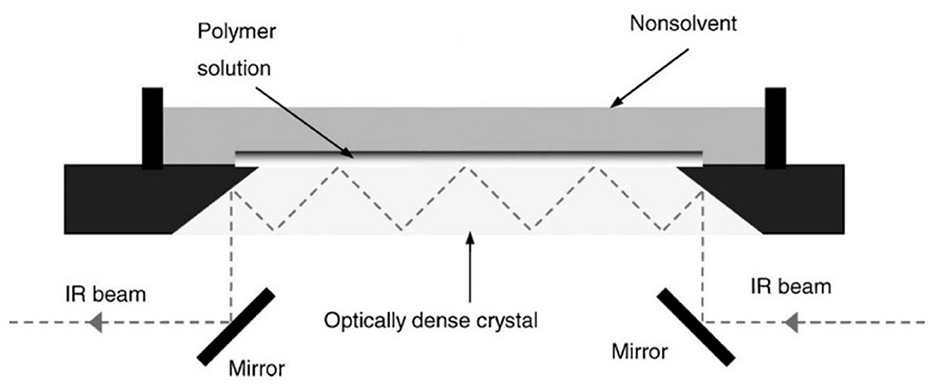 Figure 8. Schematic representation of the cross-section of the ATR-FTIR measurement cell with bottomless liquid cell for the investigation of NIPS. For EIPS, the liquid cell is not needed. The polymer solution is applied directly onto the ATR prism. Adapted from Karimi and Kish (2009) with permission. The ATR crystal material has a high refractive index that reduces the effective wavelength of the infrared light by retardation. This improves the spatial resolution compared to FTIRM (Sammon et al., 2002). The penetration depth of the probing radiation is limited by its wavelength and the refractive indices of the ATR crystal and the polymer solution, resulting in an effective penetration depth of < 5 μm. The change of the refractive index of the polymer solution due to diffusional processes during the phase separation process also needs to be considered. The temporal resolution is 0.2 s. With a fixed penetration depth of the IR radiation, the film composition at different distances from the air-side interface is measured by applying the polymer solution with varying thickness. During TIPS, ATR-FTIR enables the measurement of concentration gradients along the cross-section of cast film, thus overcoming the main limitation of the gravimetric method discussed in Section 2.1.3.1 (Zeman and Fraser, 1994). During NIPS, the composition path of specific horizontal planes inside the cast film is determined by measuring the change of the ratio of solvent and non-solvent content. A ratio >1 implies a higher solvent outflow than non-solvent inflow. This is associated with a sponge-like membrane morphology, whereas a ratio < 1 is associated with a finger-like structure (Karimi and Kish, 2009). The diffusivity of various non-solvents investigated with ATR-FTIR ranks in the following order: water > methanol > ethanol > n-propanol > n-butanol. Decreasing diffusivity is associated with a suppression of macrovoids and a change from defect-free to defective skins (Fan et al., 2002). After the membrane formation process is complete, residual substances, e.g. solvent, remain in the membrane, with increasing concentration toward the support-side interface. These may cause redissolution and redemixing of the polymer during the drying step, if the membrane is not rinsed properly. With ATR-FTIR, the presence and the concentration of residual substances is detectable (Tsai et al., 2009). 2.1.3.2.3. Micro-Raman spectroscopyAnother spectroscopic technique to analyse the chemical composition of a polymer solution is micro-Raman spectroscopy (MRS). A possible experimental setup combines a laser as a light source with a CLSM as sample carrier. Such a CLSM setup has a focalization cylinder of 1 mm in diameter and 6 mm in depth (Menut et al., 2008). MRS is a promising technique to investigate diffusion processes close to the air-side interface by quantifying the composition on the side of the coagulation bath and on the side of the polymer solution. During NIPS, the exchange rate of solvent and non-solvent through the air-side interface can be correlated with the extend of skin formation and show how this in turn influences the membrane morphology (Kim et al., 2000). During VIPS, the measurement of the change of composition at the air-side interface with time shows how dry and humid air affect the membrane morphology (Menut et al., 2008). Compared to FTIRM, MRS has an increased spatial resolution but a reduced sensitivity. For instance, it cannot detect low quantities of water. Because of a similar experimental setup as FTIRM, MRS also suffers from the same limitation, mentioned in Section 3.1.3.2.1 (Andersen and Muggli, 1981; Menut et al., 2008; Nakao et al., 2021). 2.1.4. Overview of experimental methodsIn an attempt to condense the information presented in the foregoing sections further, Table 1 summarizes the experimental methods. Each method is analyzed with regard to what kind of information it can retrieve and how its spatiotemporal resolution is. TABLE 1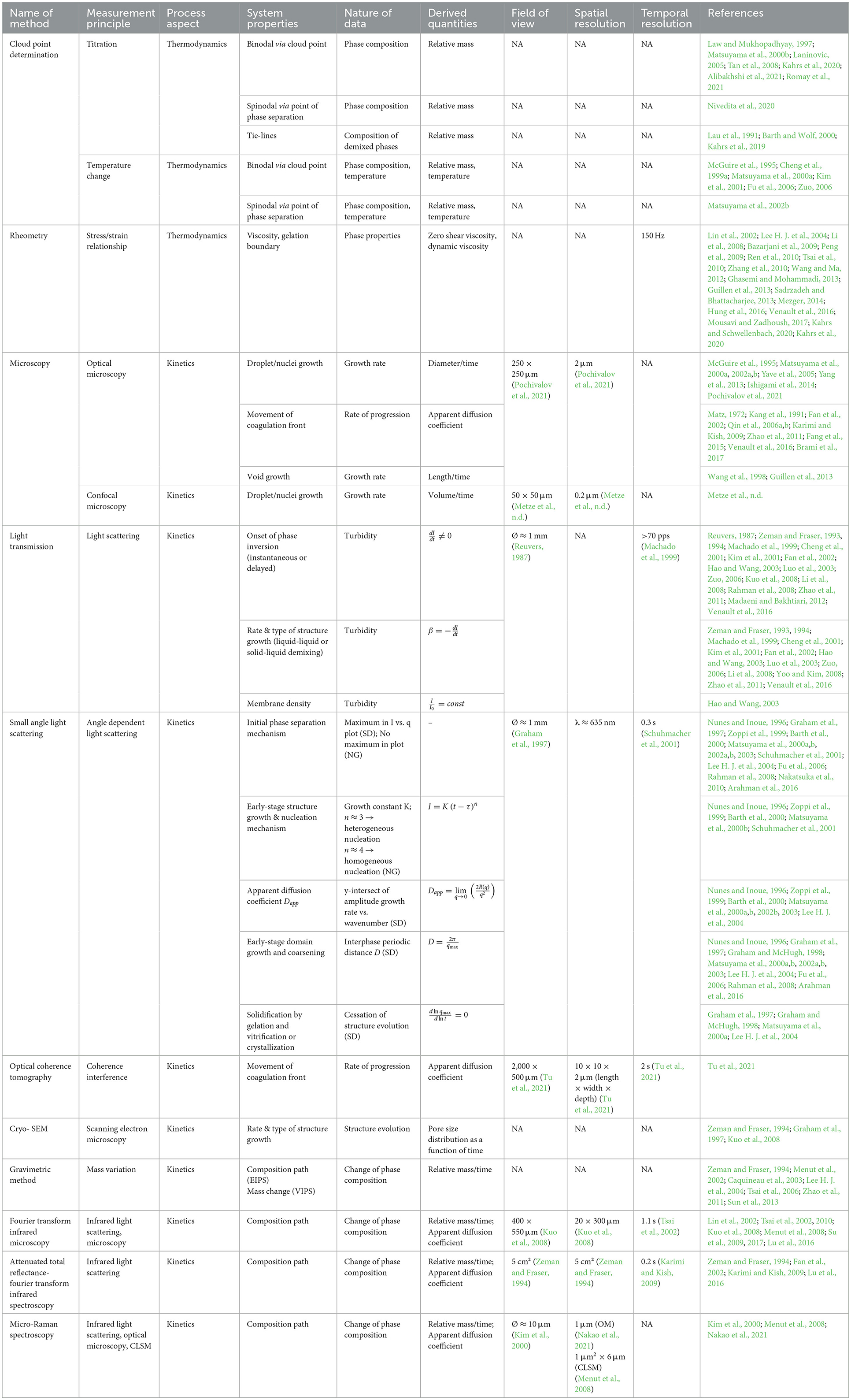
Table 1. Summary of experimental methods for the characterization of membrane formation processes. 2.2. Computational methods for membrane formation characterizationIn the following section, a general overview of the computational methods for membrane formation characterization will be given. The approaches are categorized according to their spatiotemporal resolution ranging from macroscopic to microscopic. Subsequently, the mesoscopic phase-field method will be elucidated further, since its ability to resolve the microstructure evolution of membrane formation in the μm scale for up to several seconds makes it especially valuable approach. Macroscopic transport models are continuum models that describe mass and heat transfer and associated changes in phase composition during phase separation processes, going back to the 1970's (Anderson and Ullman, 1973). Depending on the investigated phase separation process, various combinations of mass and heat transfer need to be considered by the model, as depicted in Figure 1. A model for TIPS usually only considers heat transfer (Matsuyama et al., 1999; Li et al., 2006). EIPS and NIPS (McHugh and Yilmaz, 1985; Yilmaz and McHugh, 1986, 1988; Tsay and McHugh, 1990; Lee et al., 2010; Khansary et al., 2017) usually only involve mass transfer under isothermal conditions, whereas VIPS (Tsay and McHugh, 1991; Shojaie et al., 1994a,b; Tan et al., 1995; Khare et al., 2005; Bouyer et al., 2010; Bouyer and Pochat-Bohatier, 2013) couples heat and mass transfer, due to latent heat effects during condensation and/or evaporation. Figure 9 shows a state-of-the-art calculation of a ternary phase diagram with numerically calculated paths, initially representing the average film composition and after demixing the relative compositions of the polymer-rich and polymer-poor phase. FIGURE 9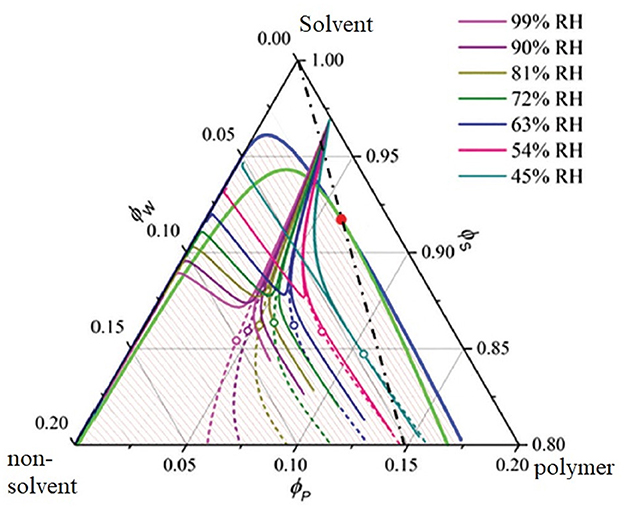 Figure 9. A ternary phase diagram of a PVDF/cyclohexanone/water system undergoing VIPS. The colored lines emerging from a single point on the solvent axis indicate the numerically simulated composition trajectories associated with different relative humidity. Demixing occurs at the points where the solid and the dashed lines split and the open symbols (∘) indicate the phase separation. Adapted from Dehsari et al. (2017) with permission. Initially, computational modeling of membrane formation was a means to understand the underlying processes in their mechanistic details and to predict the effects of parameter variation and not for a priori predictions of membrane properties with substance data & boundary conditions (i.e., process parameters) alone. 2.2.1. Scope of microscopic and mesoscopic computational approachesIn contrast to the top-down approach of macroscopic transfer models, microscopic molecular or particle based computational approaches such as molecular dynamics (MD) (Bhattacharya et al., 1998; Lee, 1999; Gee et al., 2006; Luo and Jiang, 2010; Zausch et al., 2010; Liu et al., 2011; Singh et al., 2014), dissipative particle dynamics (DPD) (He Y.-D. et al., 2011; Tang et al., 2016; Wang et al., 2017; Lin et al., 2018; Jiang et al., 2020) and Monte Carlo (MC) (Torrie and Valleau, 1977; Hayward et al., 1987; Yaldram and Binder, 1991; Huang and Feigenson, 1993; Glotzer et al., 1994; Lomakin et al., 1996), as well as mesoscopic approaches including self-consistent field (SCF) model (Glotzer, 1994; Müller and Binder, 1998; Drolet and Fredrickson, 1999; Reister et al., 2001; Ceniceros and Fredrickson, 2004; Sides and Fredrickson, 2004; Wang et al., 2004, 2008; Zhang et al., 2011; Sandhu et al., 2013) and phase-field (PF) models (Badalassi et al., 2003; Wang et al., 2012, 2019, 2020; Kouijzer et al., 2013; Tree et al., 2017; Ronsin et al., 2020), use a bottom-up approach. MD simulations are usually on the atomistic scale and the calculation is based on Newton's second law of motion by properly formulating the potentials for polymer solutions, such as Weeks-Chandler-Andersen (WCA) potential (Heyes, 2007; Ahmed and Sadus, 2009; Zhou et al., 2020), Lennard-Jones (LJ) potential (Koura and Matsumoto, 1991; Bouanich, 1992; Smit, 1992; Semiromi and Azimian, 2011; Geada et al., 2018), and finite extensible non-linear elastic (FENE) potential (Hyon and Liu, 2010; Barrett and Süli, 2011; Morthomas et al., 2017). DPD also employs Newton's second law. The concept of a particle representing a cluster of monomers is adopted in this method and the corresponding simulations predict microstructural evolution of membranes on the mesoscopic scale (Hu et al., 2017) (see Figure 10A). In contrast to the conventional all-atom MD simulation, coarse grain MD (Saiz et al., 2002; Loison et al., 2004; Wohlert et al., 2006; Bond et al., 2007; Brocos et al., 2012; Hakobyan and Heuer, 2013; Periole, 2017; Crespo et al., 2020) has been developed to compute membrane structures at scales larger than atomistic length (see Figures 10B–D). The concept of coarse grain MD is similar to DPD, where a rigid-body nanoparticle is used to represent several beads of polymer monomers. FIGURE 10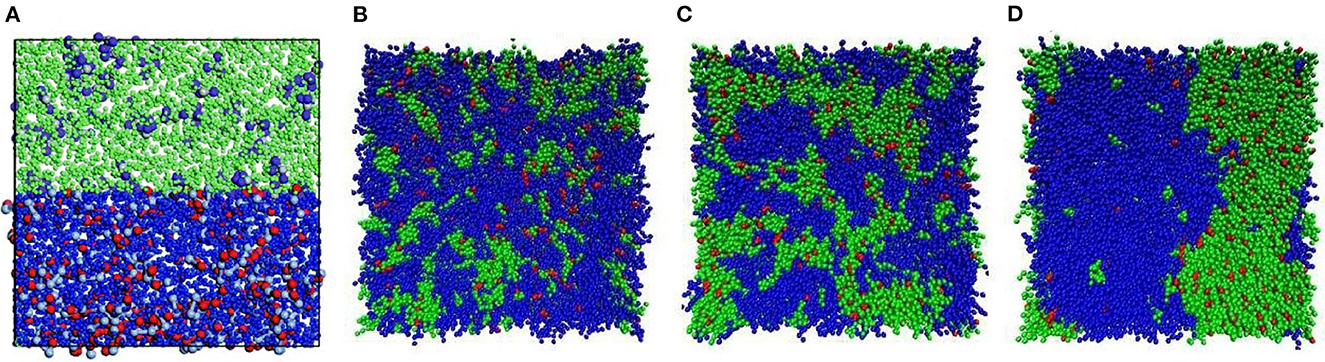 Figure 10. (A) DPD simulation for a membrane structure consisting of hexane (green), water (blue), TMC (violet), and BDSA (red). Adapted from Hu et al. (2017) with permission. (B–D) Coarse grain MD simulations for a membrane bilayer consisting of DPPC (green), DUPC (blue), and CHOL (red) at the time 0, 100 ns, and 12 s, respectively. Reproduced from Hakobyan and Heuer (2013) with permission. In contrast to the use of deterministic equations to simulate the membrane structure in MD and DPD, the random sampling method MC has sometimes been used to simulate the deterministic behavior of microstructural evolution of membranes. Depending on the sampling object, one monomer or a bead consisting of several monomers, there are atomistic and mesoscopic MC approaches (Dodd and Theodorou, 1994; Pant and Theodorou, 1995; Groot, 2000; Yamamoto and Hyodo, 2003; Deserno, 2009; Groves et al., 2010; Pandey and Doxastakis, 2012). The last two methods, SCF and PF models are a kind of mean-field theory within the context of thermodynamics. Herein, a local polymer composition field φ = (φ1, φ2, ⋯ ,φK) is introduced to formulate the free energy functional F(φ) of the system. The notation φi represents the composition of the i-species in the polymer solution and K denotes the total number of species. An often-used assumption in the mean-field theory is a Gaussian chain for the polymer beads (Flory, 1942, 1953; Sariban and Binder, 1987; Shull and Kramer, 1990). The membrane structure is obtained by minimizing the free energy functional. In contrast to atomistic methods, the benefit of the mean-field methods is to simulate the microstructural formation of membranes at comparatively large time scales, where the effect of hydrodynamics comes into play. A summary of the computational methods for modeling the formation of polymer membranes is tabulated in Section 2.2.3. In the following, we will particularly review the application of the phase-field method to simulate the formation of membrane structures. There are two types of phase-field approaches in literature for the study of membrane structure evolution. The first one is the Allen-Cahn-type phase-field model (Allen and Cahn, 1972, 1979) formulated for non-conserved order parameters. This model is rarely used to investigate the formation of membrane structures (De Masi, 1994; Capuzzo Dolcetta et al., 2002; Shen and Yang, 2010; Lee and Lee, 2014). The second one is the Cahn-Hilliard-type phase-field model elaborating conserved field variables, which is the most common one for the study of membrane structures (Cahn and Hilliard, 1958; Pego, 1989; Lowengrub and Truskinovsky, 1998; Dai and Promislow, 2013; Zheng et al., 2015; Avalos et al., 2016; Cabral and Higgins, 2018; Hopp-Hirschler et al., 2019). This approach is usually coupled with the Flory-Huggins (FH) model, which is a mean-field method to formulate the free energy density of polymer solutions. The advantages and disadvantages of the FH model for polymer solutions have been discussed in Donnelly et al. (2015), Jamali et al. (2015), Nguyen et al. (2017), and Loo et al. (2019). The FH free energy density is expressed as f(φ)=kbT∑i=1KφilnφiNi+∑i |
【本文地址】
今日新闻 |
推荐新闻 |