3.1: Introduction to stereochemistry |
您所在的位置:网站首页 › stereochemistry音标 › 3.1: Introduction to stereochemistry |
3.1: Introduction to stereochemistry
|
Stereoisomers
Stereoisomers have the same sequence of bonds but the atoms or groups of atoms are oriented differently in space.
Conformers or conformational isomers are the stereoisomers in which the different orientations of atoms are a result of rotation around single bonds. The specific arrangement of atoms in a conformational isomer is also called conformation.
Configurational isomers are stereoisomers that can be interconnected only by breaking and making some bonds. The specific arrangement of atoms in a configuration isomer is also called configuration.
Conformers or conformational isomers
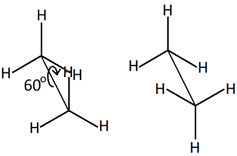
Conformers are the isomers that are the result of rotation around single bonds. They are also called different conformations of the same molecule. For example, rotation around \(\ce{C-C}\) bond of ethane places a set of three \(\ce{H's}\) on one \(\ce{C}\) at different positions relative to \(\ce{H's}\) on the other \(\ce{C}\), as illustrated below.
Different arrangements of \(\ce{H's}\) in ethane due to rotation around \(\ce{C-C}\) bond
Illustration of rotation around \(\ce{C-C}\) bond in ethane (Copyright; mailto:[email protected], CC BY 2.5 via Wikimedia Commons) Usually, the rotation around single bonds happens rapidly at room temperature. So, the conformers usually exist as a mixture and can not be easily separated. Configurational isomersConfigurational isomers are stereoisomers that can be interconnected only by breaking and making some bonds. For example, cis-but-2-ene and trans-but-2-ene are configurational isomers in which \(\ce{-CH3}\) groups connected to two \(\ce{C's}\) of a double bond are oriented differently as shown in Figure \(\PageIndex{1}\). Another example is L-glyceraldehyde and D-glyceraldehyde in which four different groups are connected to the same \(\ce{C}\) but oriented differently as shown in Figure \(\PageIndex{1}\). 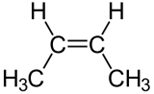 Cis-but-2-ene Cis-but-2-ene
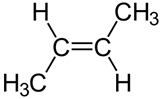 Trans-but-2-ene Trans-but-2-ene
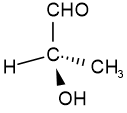 D-glyceraldehyde D-glyceraldehyde
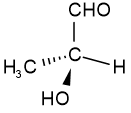 L-glyceraldehyde
Figure \(\PageIndex{1}\): Examples of configurational isomers: cis- and trans-but-2-ene, and D- and L-glyceraldehyde. (Copyright; Public domain). L-glyceraldehyde
Figure \(\PageIndex{1}\): Examples of configurational isomers: cis- and trans-but-2-ene, and D- and L-glyceraldehyde. (Copyright; Public domain).
The configurational isomers are subdivided into enantiomers and diastereomers. EnantiomersA pair of stereoisomers that are related to each other as non-superimposable mirror images are Enantiomers. For example, D-glyceraldehyde and L-glyceraldehyde shown above are enantiomers of each other. Imagine there is a mirror between D- and L-glyceraldehyde shown above. You will notice that they are mirror images of each other. If you try to overlap L-glyceraldehyde onto D-glyceraldehyde, two groups may overlap, but the other two will not overlap, no matter how you may rotate the molecule. D- and L- represent two different configurations of glyceraldehyde shown in Figure \(\PageIndex{1}\). Enantiomers are chiral molecules. ChiralityAn object or molecule that cannot be superimposed on its mirror image by any translation, rotational, or conformational changes is a chiral object. This geometric property is called chirality. Achiral is not chiral, i.e., the objects or molecules that are identical to their mirror image are achiral. For example, an amino acid with four different groups attached to the same carbon and hand are chiral, having a non-superimposable mirror image as illustrated in Figure \(\PageIndex{2}\). Like left and right hands that have a thumb and fingers in the same order, but are mirror images and not the same, chiral molecules have the same things attached in the same order, but are mirror images and not the same. 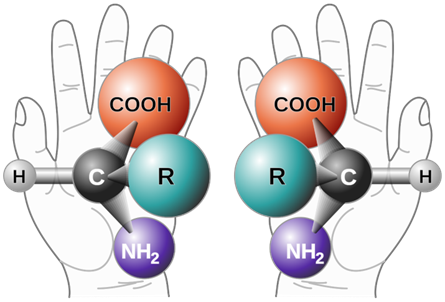 Figure \(\PageIndex{2}\): Illustration of chiral objects and their non-superimposable mirror images -a hand and an amino acid with four different groups attached to the same \(\ce{C}\). (Copyright; πϵρήλιο, Public domain, via Wikimedia Commons)
Diastereomers
Figure \(\PageIndex{2}\): Illustration of chiral objects and their non-superimposable mirror images -a hand and an amino acid with four different groups attached to the same \(\ce{C}\). (Copyright; πϵρήλιο, Public domain, via Wikimedia Commons)
Diastereomers
Stereoisomers that are not enantiomers are diastereomers. For example, cis-but-2-ene and trans-but-2-ene shown in Figure \(\PageIndex{1}\) are diastereomers because they have the same formula, and the same atom-connectivity, but methyl groups are oriented in the same direction in cis- and in opposite directions in trans-isomer, they do not mirror each other. The cis- and trans- represent two different configurations of but-2-ene as shown in Figure \(\PageIndex{1}\). |
【本文地址】
今日新闻 |
推荐新闻 |