猪流行性腹泻(PED):对一种老疾病的新认识 |
您所在的位置:网站首页 › g病毒形态图 › 猪流行性腹泻(PED):对一种老疾病的新认识 |
猪流行性腹泻(PED):对一种老疾病的新认识
|
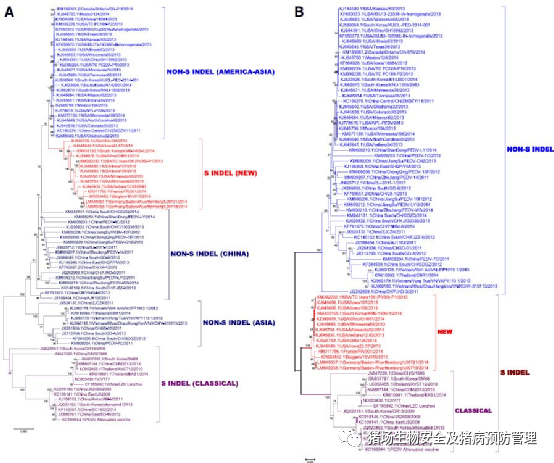
Distribution:分布 Porcine Epidemic Diarrhoea Virus(PEDV):猪流行性腹泻病毒 Alpha:α冠状病毒 Acute and watery diarrhoea in pigs of all ages:所有年龄段猪只急性感染和水样腹泻 Mortality can reach up to 100 % in suckling piglets of less than 2 weeks due to severe dehydration:2周龄以下的哺乳仔猪因严重脱水,死亡率可达100% Only sporadic outbreaks in Europe during the last 10 years but a relevant cause of diarrhoea in pig farms in Asia since the 80s. Firstly described in America in 2013:过去10年欧洲仅零星爆发,但自80年代以来在亚洲猪场出现。2013年在美国首次爆发。 Transmissible Gastroenteritis Virus(TGEV):传染性胃肠炎病毒 Alpha:α冠状病毒 Enteric disease clinically indistinguishable of porcine epidemic diarrhoea:与猪流行性腹泻临床上无法区分的肠道疾病 Only very sporadic outbreaks in countries where PRCV is widespread:在出血热广泛流行的国家,只有零星的爆发 Porcine Respiratory Coronavirus (PRCV):猪冠状病毒 Alpha:α冠状病毒 Self-limiting respiratory infection. Often subclinical but can exacerbate respiratory symptoms caused by other pathogens:自限性呼吸道感染。通常为亚临床感染,但可加重由其他病原体引起的呼吸道症状 Endemic infection in many European swine herds:许多欧洲猪群的地方性感染 Hemagglutinating Encephalomyelitis Virus (HEV):戊型肝炎病毒 Beta:β冠状病毒 Neurotropic virus causing the typical vomiting and wasting disease or acute encephalomyelitis with motor disorders in piglets:引起仔猪典型呕吐和消耗性疾病或急性脑脊髓炎并伴有运动障碍的嗜神经病毒 Widespread infection although most of the cases remain subclinical:广泛感染,尽管大多数病例仍处于亚临床状态 Porcine Delta Coronavirus (PDCoV):德尔塔冠状病毒 Delta:δ冠状病毒 Mild to moderate enteric disease in young piglets similar to porcine epidemic diarrhoea or transmissible gastroenteritis:与猪流行性腹泻、传染性肠胃炎相似的仔猪轻度至中度肠道疾病 First identified in Hong Kong, China, in 2009 and North America in early 2014. However, a recent research detected anti-PDCoV IgG antibodies in serum samples collected in 2010, indicating an earlier undetected presence of PDCoV in the US pig population:2009年首次在中国香港发现,2014年初在北美发现。然而,最近的一项研究在2010年收集的血清样本中检测到了抗PDCoV IgG抗体,这表明在美国猪群中出现了更早的未被检测到的PDCoV。 图1:基于地理和时间标准选择PEDV全基因组(a)和S基因(b)对核苷酸序列进行遗传进化树分析
Infection sources and transmission Direct and indirect PEDV transmission occurs mainly by faecal-oral route. Viral shedding in faeces starts on postinfection day one or two and continues for a period of 7 to 10 days [35, 36], although it can extend up to 36 weeks in some animals [37, 38]. The transmission of the infection is facilitated by the high viral load in faeces from infected animals [39, 40] as well as by the minimum infectious dose required to infect naïve pigs [31]. Moreover, the resistance of the virus in the environment facilitates the faecal-oral transmission. PEDV is stable under low temperatures, while it is adversely affected by high temperatures. It survives between pH 5.0–9.0 at 4 °C while only between pH 6.5–7.5 at 37 °C. It can survive for at least 28 days in slurry at 4 °C, 7 days in contaminated dry feed at 25 °C or 14 days in contaminated wet feed at 25 °C [31]. This fact favours the indirect transmission by different faeces-contaminated fomites such as transport vehicles [41], feed [42], clothing or footwear. Genetic and phylogenetic analyses of American PEDV isolates revealed a close relationship with Chinese isolates and their likely Chinese origin [43]. However, how the virus might have travelled from China to the USA is a matter of speculation. The rapid spread of PEDV on swine farms in the USA raised questions regarding the possibility of airborne transmission of this infection. Although undoubtedly the faecal-oral route is the main source of PEDV transmission, it has been suggested [44] that PEDV may travel through the air for short distances on faecal dust particles, at least under certain conditions. However, airborne transmission of PEDV has only been shown under experimental conditions and up to now infectious PEDV has not been demonstrated in field air samples containing PEDV genetic material [44, 45]. The role that vectors play in the transmission of PEDV has also been investigated. So far, there has been no evidence of PEDV replication in non-porcine hosts, including rodents and starlings [46–48]. However, the potential role of vectors in the mechanic transmission of the virus from one farm to another cannot be ruled out, as has been described for TGEV [4]. Using highly sensitive molecular assays the presence of viral RNA has been reported in milk samples from infected lactating sows [28, 29] as well as in semen samples [29, 31]. However, infectious PEDV in these samples has not been demonstrated and their contamination with faecal material in the sampling cannot be excluded. Moreover, viral RNA has been detected in the serum fraction of whole blood samples from infected pigs [40, 49]. The role of spray-dried porcine plasma (SDPP), normally used as feed additive, as a potential vehicle of transmission of PEDV has been researched into. A number of experimental studies have demonstrated that spray-drying process as well as storage conditions are sufficient to inactivate infectious PEDV in SDPP [50, 51]. The infectivity of commercial SDPP positive for PEDV-RNA has also been investigated. A research group from Canada managed to reproduce PEDV infection in SDPP-inoculated piglets, although they failed to reproduce the infection in animals receiving feed supplemented with the same PEDV-positive SDPP [52]. Similarly, neither clinical signs nor PEDV RNA in faeces or PEDV specific antibodies were detected in pigs which were fed a diet containing 5 % SDPP confirmed positive for PEDV, in a bioassay experiment conducted by Opriessnig et al. [53]. According to this, there is no experimental evidence of PEDV transmission through PCR positive SDPP supplemented feed. This experimental data is corroborated by the fact that despite the use of large amounts of PEDV positive SDPP from the USA to feed pigs in Brazil or Western Canada, these areas remained free of PEDV infection [54]. 感染源和传播 PEDV主要通过粪口途径直接和间接传播。病毒在粪便中的排毒始于感染后的第1-2天,并持续7-10天,但在某些猪只中可延长至36周。受感染猪只粪便中的高病毒载量以及感染阴性猪所需的最低感染剂量,促进了感染的传播。此外,病毒在环境中的耐性促进了粪口传播。PEDV在低温下是稳定的,但在高温下会受影响。在4℃ pH值5.0-9.0之间、37℃ pH值6.5-7.5之间存活。在4℃的泥浆中至少能存活28天,在25℃污染的干饲料中至少能存活7天,在25℃污染的湿饲料中至少能存活14天。这有利于通过交通工具、饲料、服装、鞋类等粪便污染物的间接传播。 美国PEDV分离株的遗传和系统发育分析表明,该分离株与中国PEDV分离株关系密切,可能源自中国。然而,病毒是如何从中国传播到美国? PEDV在美国猪场的迅速传播提出了关于这种感染可能通过空气传播的可能。尽管粪口途径是PEDV传播的主要来源,但有人认为PEDV至少在某些条件下可以通过粪便尘埃颗粒在空气中短距离传播。然而,PEDV的空气传播仅在实验条件下被证明。调查了病媒在PEDV传播中的作用。到目前为止,尚未发现PEDV在非猪宿主中复制的证据。但是,不能排除病媒在病毒从一个猪场机械传播到另一个猪场的潜在作用。 使用高度敏感的分子检测方法,已报道在感染哺乳母猪的常乳和精液中存在病毒RNA。然而,这些样本中传染性PEDV尚未被证实,不能排除其被采样中的粪便污染。此外,已在感染猪只全血样本的血清中检测到病毒RNA。 研究了猪喷雾干燥血浆蛋白(SDPP)作为PEDV潜在传播媒介的作用。大量实验研究表明,喷雾干燥过程和储存条件足以灭活SDPP中感染性PEDV。对PEDV阳性SDPP传染性也进行了研究。来自加拿大的一个研究小组成功地在接种了SDPP的仔猪中复制了PEDV感染,尽管他们未能在喂食了相同的PEDV阳性SDPP猪只中复制感染。同样,Opriessnig等人进行的一项生物测定实验表明,在喂食含有5% SDPP饲粮的猪只中,证实PEDV阳性的猪只既没有出现临床症状,也没有发现粪便中PEDV RNA,也没有发现PEDV特异性抗体。由此可见,没有实验证据表明PEDV通过PCR阳性SDPP传播。尽管巴西或加拿大西部地区使用了大量来自美国的PEDV阳性SDPP饲料喂养猪只,但这些地区仍未发现PEDV感染,这一实验数据得到了证实。 Pathogenesis, clinical signs and lesions PEDV replicates in the cytoplasm of villous enterocytes of the small intestine and causes villous shortening and reduced enzymatic and absorptive capacity in the small intestine causing profuse watery diarrhoea, which lasts about a week [37, 55, 56]. Other clinical signs which are frequently associated to PEDV infection include vomiting, anorexia and fever. Although pigs of all ages are affected, the severity of PED is higher in suckling piglets of less than one week old which may die due to severe dehydration. The slower turnover of enterocytes in neonatal piglets (5–7 days) compared to three weeks-old piglets (2–3 days) could explain, at least partially, the higher susceptibility of these young piglets to PEDV [4]. PEDV has also been detected in epithelial cells of the colon in both experimentally and naturally infected pigs, although villous atrophy has not been demonstrated in the large intestine [40]. Replication of PEDV was classically circumscribed to the intestinal tract [3], until a recent research showed PEDV replication in alveolar macrophages of 3 day-old-colostrum-free piglets, which were experimentally inoculated with a Korean wild-type PEDV isolate [57]. Further studies are needed to confirm whether extra-intestinal replication also occurs with other PEDV isolates as well as to determine their clinical and epidemiological relevance. Two epidemiologic presentations of PED have been described on the farms. (a) Epidemic PED outbreaks occur when PEDV is introduced into a naïve farm (where most of the animals are PEDV seronegative). The disease spreads rapidly affecting pigs of all ages with morbidity approaching 100 %. Moreover, PEDV can persist and become (b) endemic on the farm affecting post-weaning piglets that have lost their lactogenic immunity as well as newly introduced seronegative gilts. Mortality associated with PED outbreaks is highly dependent on the age of the infected animals. Mortality can reach up to 80–100 % in suckling piglets of less than one week old, while in weaned pigs mortality rates are typically only 1 to 3 % [11, 30]. No mortality associated with PED is usually observed among adult pigs. As has already been mentioned, differences in the severity of PED outbreaks have been reported. Particularly severe PED outbreaks have been described in Asia since 2010 and also in the USA. Differences in the virulence of PEDV isolates have been proposed to explain this variability [28, 58, 59]. From our point of view, this is one of the most relevant questions to face regarding PED nowadays: the reason or reasons which could explain variations in the clinical outcome of an outbreak. Although some reports have suggested that they could be associated with differences in the virulence of PEDV isolates, exhaustive challenge studies using pig adapted virus (not cell culture adapted isolates) in suckling piglets are needed to elucidate the role of the strain. Some insights have been obtained related to the virulence of different strains. In the USA, at least two main variants of PEDV have been recently identified using molecular methods. The first one seems to be a highly virulent virus and similar to viruses described in several Asian countries after 2010 while the second, the S INDEL variant, has been associated to mild clinical outbreaks [59]. This S INDEL variant includes some particular insertions and deletions in the S gene and is also similar to some Asian isolates, part of which were recovered before 2010. The classical European reference strain of PEDV CV777 is also an S INDEL isolate although it is located in a different cluster and well differentiated from American INDEL isolates (Fig. 1a and b). PEDV isolates recovered in European countries (Germany, Italy, Belgium, the Netherlands and France) in 2014 and 2015 have been characterized and all of them were found to be INDEL isolates similar to the variant described in the USA [13–19]. Most of these recent PED outbreaks in Europe occurred in fattening farms and, as expected, no mortality was observed. However, PEDV isolates recently recovered in severe outbreaks of PEDV in Ukraine have shown a genome nucleotide similarity reaching 99.8 % with non-INDEL isolates from the United States and Mexico [20]. So far, this has been the only report of PEDV non-INDEL isolates in Europe. Apart from differences in the virulence of the PEDV strains, many other parameters including management, immune status of the population and herd sanitary status could also explain variations in the clinical outcome of PED outbreaks [31]. Thus, the contribution of co-infections with other viruses, particularly with other enteric viruses such as porcine delta coronavirus (PDCoV) or the recently described mammalian orthoreovirus 3 (MRV3) has also been pointed out. Both viruses have been detected in faecal samples collected from PEDV positive farms in the USA. PDCoV has been associated with mild to moderate diarrhoea in experimentally inoculated naïve suckling piglets [33] while MRV3 caused severe diarrhoea with 100 % mortality in 3-day-old piglets [60]. 发病机制、临床体征及病变 PEDV在小肠绒毛肠细胞的细胞质中复制,导致小肠绒毛缩短,酶和吸收能力降低,导致大量水样腹泻,持续约一周。通常与PEDV感染有关的其他临床症状包括呕吐、厌食和发烧。尽管所有年龄段的猪都受感染,但PED的严重程度在不到一周的哺乳仔猪中较高,这些仔猪可能会因严重脱水而死亡。与3周龄仔猪(2-3日龄)相比,新生仔猪(5-7天)肠上皮细胞更替较慢,这至少部分解释了这些仔猪对PEDV的较高易感性。 在实验和自然感染猪的结肠上皮细胞中也检测到PEDV,尽管在大肠中未发现绒毛萎缩。 PEDV的复制通常局限于肠道,直到最近的一项研究表明,PEDV在3日龄未吃初乳的仔猪肺泡巨噬细胞中复制,实验中接种了韩国PEDV野毒株。需要进一步的研究来确认是否其他PEDV分离株也会发生肠外复制,以及确定它们的临床和流行病学相关性。PED的两种流行病学表现已在猪场上进行了描述。(a)当PEDV被引入阴性猪场时,就会发生流行性PEDV爆发。该疾病传播迅速,对所有年龄的猪均有影响,发病率接近100%。此外,PEDV可持续存在并在猪场流行,影响断奶后失去乳源性免疫的仔猪以及新引进的血清阴性母猪。 PED爆发相关的死亡率高度依赖于受感染猪只日龄。不到一周的哺乳仔猪死亡率可达80%-100%,而断奶仔猪的死亡率通常只有1%-3%。在成年猪中,通常未观察到与PED相关的死亡。 如前所述,PED疫情的严重程度有所不同。自2010年以来,在亚洲和美国都出现了特别严重的PED疫情。PEDV分离株毒力的差异被认为可以解释这种差异。从我们的观点来看,这是当今PED面临的最相关的问题之一:一个或多个可以解释爆发临床结果差异的原因。尽管一些报告表明,它们可能与PEDV分离株的毒力差异有关,但需要在哺乳仔猪中使用猪适应病毒(而不是细胞培养适应的分离株)进行全面的攻毒研究,以阐明该毒株的作用。 已经获得了一些与不同毒株毒力有关的见解。 在美国,最近至少有两种主要的PEDV变种被分子方法鉴定出来。第一种病毒似乎是一种高毒力病毒,与2010年后在几个亚洲国家的病毒相似,而第二种病毒,即S INDEL变种,与温和的临床爆发有关。这种S INDEL变异包括S基因的一些特定的插入和缺失,也类似于一些亚洲分离株,其中部分在2010年前被发现。PEDV CV777也是INDEL分离株虽然处于不同分支(图1a和b)。对PEDV分离株在欧洲国家(德国、意大利、比利时、2014年和2015年对荷兰和法国)进行了鉴定,发现它们均为INDEL分离株,与美国变异株相似。欧洲最近爆发的PED大多发生在育肥场,如预期的那样,没有观察到死亡。然而,最近在乌克兰严重爆发的PEDV中发现的PEDV分离株与来自美国和墨西哥的非INDEL分离株的基因组核苷酸相似性达到99.8%。到目前为止,这是欧洲唯一一份关于PEDV非INDEL分离株的报告。除了PEDV毒株的毒力差异外,许多其他参数,包括管理、猪群免疫状况和群体卫生状况也可以解释PED爆发的临床结局差异。因此,也有人指出了与其他病毒,特别是与其他肠道病毒(如PDCoV或MRV3)混合感染。从美国PEDV阳性猪场收集的粪便样本中已检测到这两种病毒。在实验接种的阴性哺乳仔猪中,PDCoV与轻度至中度腹泻有关,而MRV3在3日龄仔猪中引起严重腹泻,死亡率为100%。 Control and prevention There is no specific treatment for PEDV other than supportive care and symptomatic treatment. Mortality occurs in suckling piglets as a result of dehydration which should be corrected using oral electrolyte solutions. In adult pigs, dry feed should be withdrawn for a period of 12–24 h and then, carefully reintroduced while water should be kept freely available [3, 4]. In order to increase passive immunity to piglets and minimize losses, sows due to farrow in at least 2 weeks can be deliberately exposed to virulent virus by the oral route. A recent study revealed that morbidity was reduced from 100 to 43 % in litters exposed to virulent PEDV when their sows were previously exposed to a mild virulent strain (S INDEL variant) of PEDV [63]. Oral administration of chicken egg-yolk or cow colostrum containing PEDV immunoglobulins could offer an immunoprophylactic defence [64, 65]. The increase in lactogenic immunity is also the aim of PEDV vaccines which are used in pregnant sows. Attenuated or killed vaccines against PEDV have been used in several Asian countries for years [66].However, it has been suggested that live vaccines can revert to virulence and their use and usefulness under field conditions have been questioned [5, 27, 67]. Recently, a PEDV subunit vaccine based on the S protein gene of PEDV as well as a vaccine with killed virus have been licensed in the USA [68], although there are still no studies which prove their efficacy. However, PEDV vaccines have never been used in Europe as the disease was not of sufficient economic importance in this area. In general, PEDV vaccines have been reported to be useful to booster antibody response in animals that have already been infected by PEDV. As there are no specific treatments for the control and potential eradication of the disease from the herd, preventive measures which preclude the introduction of the virus or new PEDV strains in the area, country or farm are of paramount importance. Supported by the detection methods mentioned in the diagnosis, surveillance should be used to certify that trading of swine or related derivatives do not cause the spread of new strains of the virus. Lorries used in transport have been highlighted as a relevant source of transmission [41] and special attention should be paid in the effectiveness of the cleaning and disinfecting protocols to inactivate and remove the virus. At herd level, basic external biosecurity rules such as quarantine of reposition, ban the entrance of unwashed vehicles, strict visitor policies (time interval between visiting two farms, provide footwear and appropriate clothing, showers and so on) should be carried out without exception and internal biosecurity such as controlling the slurry level, carcasses disposal and carcass bin cleaning, movement of the caretakers on the farm and so on could prevent the establishment of an endemic form of the disease. Finally, many virucidal disinfectants have been shown to be effective in inactivating PEDV. Phenol, quaternary ammonium compounds, glutaraldehyde and bleach are examples of such disinfectants. Water temperature is a crucial factor and temperatures over 60 °C help to inactivate the virus. Proper cleaning and disinfecting of facilities and equipment is crucial to control PEDV. 控制和预防 除了护理和对症治疗外,PEDV没有特定的治疗方法。哺乳仔猪因脱水而死亡,应口服电解质溶液。在成年猪中,应停用干饲料12-24小时,同时应保持水的自由供应。为了提高仔猪的被动免疫力,减少损失,可以对至少2周内分娩母猪进行返饲。最近的一项研究表明,如果母猪之前接触过PEDV的一种弱化强毒株(S INDEL变种),那么暴露于强毒株的仔猪发病率从100%下降到43%。口服含有PEDV免疫球蛋白的鸡蛋黄或牛初乳可以提供免疫预防防御。提高乳源性免疫也是PEDV疫苗的目的,该疫苗用于妊娠母猪。针对PEDV的弱毒或灭活疫苗已在一些亚洲国家使用多年。然而,有人提出,弱毒疫苗可以毒力返强,它们在临床条件下的使用和有效性受到质疑。 最近,一种基于PEDV S蛋白基因的PEDV亚单位疫苗和一种灭活病毒疫苗在美国获得了许可,但仍没有研究证明其有效性。然而,PEDV疫苗从未在欧洲使用过,因为该疾病没有足够的经济重要性。一般来说,PEDV疫苗已被报道对已感染PEDV的猪只增强抗体反应是有用的。 截止目前,没有特定的治疗方法来控制和从猪群中根除这种疾病,因此,在该地区、国家或猪场防止病毒或新的PEDV毒株传入的预防措施至关重要。监测应用于证明猪或相关衍生物的交易不会导致新的病毒株的传播。用于运输的卡车被强调为相关的传播源,应特别注意清洁和消毒程序的有效性,以灭活和清除病毒。 在猪群层面,基本的外部生物安全措施,如检疫、禁止未经清洗的车辆进入、严格的访客政策(访问两个猪场之间的时间间隔、提供鞋和适当的衣服、淋浴等)应毫无例外地进行,而内部生物安全措施,如控制泥浆水平、尸体处置和尸体箱清洁、饲养员在猪场的移动等。最后要强调的是,许多消毒剂已被证明对灭活PEDV是有效的。如苯酚、季铵盐化合物、戊二醛和漂白剂。消毒液的水温是一个关键因素,超过60℃的水温有助于灭活病毒。 正确清洁和消毒设施和设备是控制PEDV的关键。返回搜狐,查看更多 |
【本文地址】